Mutational pressure by host APOBEC3s more strongly affects genes expressed early in the lytic phase of herpes simplex virus-1 (HSV-1) and human polyomavirus (HPyV) infection
- PMID: 33930088
- PMCID: PMC8115780
- DOI: 10.1371/journal.ppat.1009560
Mutational pressure by host APOBEC3s more strongly affects genes expressed early in the lytic phase of herpes simplex virus-1 (HSV-1) and human polyomavirus (HPyV) infection
Abstract
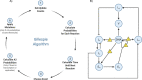
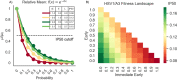
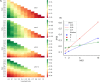
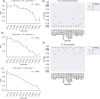
Herpes-Simplex Virus 1 (HSV-1) infects most humans when they are young, sometimes with fatal consequences. Gene expression occurs in a temporal order upon lytic HSV-1 infection: immediate early (IE) genes are expressed, then early (E) genes, followed by late (L) genes. During this infection cycle, the HSV-1 genome has the potential for exposure to APOBEC3 (A3) proteins, a family of cytidine deaminases that cause C>U mutations on single-stranded DNA (ssDNA), often resulting in a C>T transition. We developed a computational model for the mutational pressure of A3 on the lytic cycle of HSV-1 to determine which viral kinetic gene class is most vulnerable to A3 mutations. Using in silico stochastic methods, we simulated the infectious cycle under varying intensities of A3 mutational pressure. We found that the IE and E genes are more vulnerable to A3 than L genes. We validated this model by analyzing the A3 evolutionary footprints in 25 HSV-1 isolates. We find that IE and E genes have evolved to underrepresent A3 hotspot motifs more so than L genes, consistent with greater selection pressure on IE and E genes. We extend this model to two-step infections, such as those of polyomavirus, and find that the same pattern holds for over 25 human Polyomavirus (HPyVs) genomes. Genes expressed earlier during infection are more vulnerable to mutations than those expressed later.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Kennedy PG. Herpes simplex virus type 1 and Bell’s palsy—a current assessment of the controversy. J Neurovirol [Internet]. 2010. February [cited 2017 Aug 29];16(1):1–5. Available from: http://link.springer.com/10.3109/13550280903552446 - DOI - PMC - PubMed
-
- Fields BN, Knipe DM, Howley PM. Fields Virology. 6th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2013.
-
- Koonin E V., Krupovic M, Yutin N. Evolution of double-stranded DNA viruses of eukaryotes: From bacteriophages to transposons to giant viruses. Ann N Y Acad Sci [Internet]. 2015. Apr 1 [cited 2021 Mar 12];1341(1):10–24. Available from: /pmc/articles/PMC4405056/ 10.1111/nyas.12728 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

