MRI characteristics of Japanese macaque encephalomyelitis: Comparison to human diseases
- PMID: 33930224
- PMCID: PMC8722403
- DOI: 10.1111/jon.12868
MRI characteristics of Japanese macaque encephalomyelitis: Comparison to human diseases
Abstract
Background and purpose: To describe MRI findings in Japanese macaque encephalomyelitis (JME) with emphasis on lesion characteristics, lesion evolution, normal-appearing brain tissue, and similarities to human demyelinating disease.
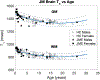
Methods: MRI data were obtained from 114 Japanese macaques, 30 presenting neurological signs of JME. All animals were screened for presence of T2 -weighted white matter signal hyperintensities; animals with behavioral signs of JME were additionally screened for contrast-enhancing lesions. Whole-brain quantitative T1 maps were collected, and histogram analysis was performed with regression across age to evaluate microstructural changes in normal appearing brain tissue in JME and neurologically normal animals. Quantitative estimates of blood-brain-barrier (BBB) permeability to gadolinium-based-contrast agent (GBCA) were obtained in acute, GBCA-enhancing lesions. Longitudinal imaging data were acquired for 15 JME animals.
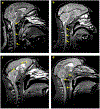
Results: One hundred and seventy-three focal GBCA-enhancing lesions were identified in 30 animals demonstrating behavioral signs of neurological dysfunction. JME GBCA-enhancing lesions were typically focal and ovoid, demonstrating highest BBB GBCA permeability in the lesion core, similar to acute, focal multiple sclerosis lesions. New GBCA-enhancing lesions arose rapidly from normal-appearing tissue, and BBB permeability remained elevated for weeks. T1 values in normal-appearing tissue were significantly associated with age, but not with sex or disease.
Conclusions: Intense, focal neuroinflammation is a key MRI finding in JME. Several features of JME compare directly to human inflammatory demyelinating diseases. Investigation of JME combined with the development and validation of noninvasive imaging biomarkers offers substantial potential to improve diagnostic specificity and contribute to the understanding of human demyelinating diseases.
Keywords: animal models; blood-brain-barrier; magnetic resonance imaging; neurodegeneration; neuroinflammation.
© 2021 American Society of Neuroimaging.
Figures






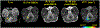


References
-
- Rooney WD, Kohama SG, Wang P, et al. MRI estimation of sub-clinical disease in Japanese macaque encephalomyelitis (abstract). Proc Int Soc Magn Reson Med 2009;17:6973.
-
- Tagge IJ, Kohama SG, Pollaro J, et al. Vascular expansion and blood-brain-barrier permeability: a comparative volumetric study in acute Japanese macaque encephalomyelitis (abstract). Proc Int Soc Magn Reson Med 2015;23:4331.
-
- Tagge I, Kohama S, Pollaro J, et al. Blood-brain-barrier permeability and lesion volume changes in acute Japanese macaque encephalomyelitis (abstract). Proc Int Soc Magn Reson Med 2015;23:0238.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

