Conductive Hydrogels with Dynamic Reversible Networks for Biomedical Applications
- PMID: 33930246
- PMCID: PMC11468162
- DOI: 10.1002/adhm.202100012
Conductive Hydrogels with Dynamic Reversible Networks for Biomedical Applications
Abstract
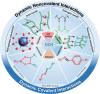
Conductive hydrogels (CHs) are emerging as a promising and well-utilized platform for 3D cell culture and tissue engineering to incorporate electron signals as biorelevant physical cues. In conventional covalently crosslinked conductive hydrogels, the network dynamics (e.g., stress relaxation, shear shining, and self-healing) required for complex cellular functions and many biomedical utilities (e.g., injection) cannot be easily realized. In contrast, dynamic conductive hydrogels (DCHs) are fabricated by dynamic and reversible crosslinks. By allowing for the breaking and reforming of the reversible linkages, DCHs can provide dynamic environments for cellular functions while maintaining matrix integrity. These dynamic materials can mimic some properties of native tissues, making them well-suited for several biotechnological and medical applications. An overview of the design, synthesis, and engineering of DCHs is presented in this review, focusing on the different dynamic crosslinking mechanisms of DCHs and their biomedical applications.
Keywords: biomedical applications; conductive hydrogels; extracellular matrix; reversible networks.
© 2021 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

