Splice site m6A methylation prevents binding of U2AF35 to inhibit RNA splicing
- PMID: 33930289
- PMCID: PMC8208822
- DOI: 10.1016/j.cell.2021.03.062
Splice site m6A methylation prevents binding of U2AF35 to inhibit RNA splicing
Abstract
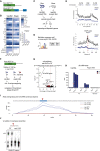
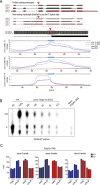
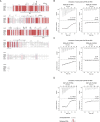
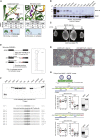
The N6-methyladenosine (m6A) RNA modification is used widely to alter the fate of mRNAs. Here we demonstrate that the C. elegans writer METT-10 (the ortholog of mouse METTL16) deposits an m6A mark on the 3' splice site (AG) of the S-adenosylmethionine (SAM) synthetase pre-mRNA, which inhibits its proper splicing and protein production. The mechanism is triggered by a rich diet and acts as an m6A-mediated switch to stop SAM production and regulate its homeostasis. Although the mammalian SAM synthetase pre-mRNA is not regulated via this mechanism, we show that splicing inhibition by 3' splice site m6A is conserved in mammals. The modification functions by physically preventing the essential splicing factor U2AF35 from recognizing the 3' splice site. We propose that use of splice-site m6A is an ancient mechanism for splicing regulation.
Keywords: 3' splice site; METT-10; METTL16; SAM homeostasis; SAM synthetase; U2AF35/65; U6 snRNA; m(6)A; spermatogenesis; splicing.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures











References
-
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. - PubMed
-
- Bailey T.L., Elkan C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1994;2:28–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

