Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study
- PMID: 33930320
- PMCID: PMC8078878
- DOI: 10.1016/S1473-3099(21)00224-3
Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study
Abstract
Background: The Pfizer-BioNTech (BNT162b2) and the Oxford-AstraZeneca (ChAdOx1 nCoV-19) COVID-19 vaccines have shown excellent safety and efficacy in phase 3 trials. We aimed to investigate the safety and effectiveness of these vaccines in a UK community setting.
Methods: In this prospective observational study, we examined the proportion and probability of self-reported systemic and local side-effects within 8 days of vaccination in individuals using the COVID Symptom Study app who received one or two doses of the BNT162b2 vaccine or one dose of the ChAdOx1 nCoV-19 vaccine. We also compared infection rates in a subset of vaccinated individuals subsequently tested for SARS-CoV-2 with PCR or lateral flow tests with infection rates in unvaccinated controls. All analyses were adjusted by age (≤55 years vs >55 years), sex, health-care worker status (binary variable), obesity (BMI <30 kg/m2vs ≥30 kg/m2), and comorbidities (binary variable, with or without comorbidities).
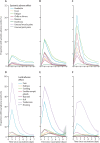
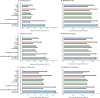
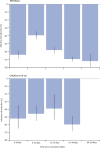
Findings: Between Dec 8, and March 10, 2021, 627 383 individuals reported being vaccinated with 655 590 doses: 282 103 received one dose of BNT162b2, of whom 28 207 received a second dose, and 345 280 received one dose of ChAdOx1 nCoV-19. Systemic side-effects were reported by 13·5% (38 155 of 282 103) of individuals after the first dose of BNT162b2, by 22·0% (6216 of 28 207) after the second dose of BNT162b2, and by 33·7% (116 473 of 345 280) after the first dose of ChAdOx1 nCoV-19. Local side-effects were reported by 71·9% (150 023 of 208 767) of individuals after the first dose of BNT162b2, by 68·5% (9025 of 13 179) after the second dose of BNT162b2, and by 58·7% (104 282 of 177 655) after the first dose of ChAdOx1 nCoV-19. Systemic side-effects were more common (1·6 times after the first dose of ChAdOx1 nCoV-19 and 2·9 times after the first dose of BNT162b2) among individuals with previous SARS-CoV-2 infection than among those without known past infection. Local effects were similarly higher in individuals previously infected than in those without known past infection (1·4 times after the first dose of ChAdOx1 nCoV-19 and 1·2 times after the first dose of BNT162b2). 3106 of 103 622 vaccinated individuals and 50 340 of 464 356 unvaccinated controls tested positive for SARS-CoV-2 infection. Significant reductions in infection risk were seen starting at 12 days after the first dose, reaching 60% (95% CI 49-68) for ChAdOx1 nCoV-19 and 69% (66-72) for BNT162b2 at 21-44 days and 72% (63-79) for BNT162b2 after 45-59 days.
Interpretation: Systemic and local side-effects after BNT162b2 and ChAdOx1 nCoV-19 vaccination occur at frequencies lower than reported in phase 3 trials. Both vaccines decrease the risk of SARS-CoV-2 infection after 12 days.
Funding: ZOE Global, National Institute for Health Research, Chronic Disease Research Foundation, National Institutes of Health, UK Medical Research Council, Wellcome Trust, UK Research and Innovation, American Gastroenterological Association.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests CM reports grants from Chronic Disease Research Foundation (CDRF) during the conduct of the study. JW, AM, LP, CH, SS, and JC report being employees of ZOE Global during the conduct of the study. ATC reports grants from Massachusetts Consortium on Pathogen Readiness during the conduct of the study, and personal fees from Bayer Pharma, Pfizer, and Boehringer Ingelheim, outside the submitted work. DAD reports grants from National Institutes of Health (NIH), Massachusetts Consortium on Pathogen Readiness, and American Gastroenterological Association, during the conduct of the study, and that he served as a co-investigator on an unrelated nutrition trial sponsored by ZOE Global. CHS reports grants from Alzheimer's Society during the conduct of the study. AMV reports grants from Medical Research Council (MRC) and personal fees from ZOE Global, during the conduct of the study. ALG reports having shares in AstraZeneca and receiving grants from Novavax, outside the submitted work. CJS reports grants from CDRF, MRC, and Wellcome Trust, during the conduct of the study. SO reports grants from Wellcome Trust, UK Research and Innovation (UKRI), and CDRF, during the conduct of the study. TDS reports being a consultant for ZOE Global, during the conduct of the study. All other authors declare no competing interests.
Figures

Comment in
-
Symptom study app provides real-world data on COVID-19 vaccines.Lancet Infect Dis. 2021 Jul;21(7):890-891. doi: 10.1016/S1473-3099(21)00264-4. Epub 2021 Apr 27. Lancet Infect Dis. 2021. PMID: 33930321 Free PMC article. No abstract available.
References
-
- Public Health England COVID-19 vaccination programme. Nov 27, 2020. https://www.gov.uk/government/collections/covid-19-vaccination-programme
-
- Department of Health and Social Care Priority groups for coronavirus (COVID-19) vaccination: advice from the JCVI, 30 December 2020. Dec 30, 2020. https://www.gov.uk/government/publications/priority-groups-for-coronavir...
-
- Hunter PR, Brainard J. Estimating the effectiveness of the Pfizer COVID-19 BNT162b2 vaccine after a single dose. A reanalysis of a study of “real-world” vaccination outcomes from Israel. medRxiv. 2021 doi: 10.1101/2021.02.01.21250957. published online Feb 3. (preprint). - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

