Evolving concepts in NAD+ metabolism
- PMID: 33930322
- PMCID: PMC8172449
- DOI: 10.1016/j.cmet.2021.04.003
Evolving concepts in NAD+ metabolism
Abstract
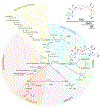
NAD(H) and NADP(H) have traditionally been viewed as co-factors (or co-enzymes) involved in a myriad of oxidation-reduction reactions including the electron transport in the mitochondria. However, NAD pathway metabolites have many other important functions, including roles in signaling pathways, post-translational modifications, epigenetic changes, and regulation of RNA stability and function via NAD-capping of RNA. Non-oxidative reactions ultimately lead to the net catabolism of these nucleotides, indicating that NAD metabolism is an extremely dynamic process. In fact, recent studies have clearly demonstrated that NAD has a half-life in the order of minutes in some tissues. Several evolving concepts on the metabolism, transport, and roles of these NAD pathway metabolites in disease states such as cancer, neurodegeneration, and aging have emerged in just the last few years. In this perspective, we discuss key recent discoveries and changing concepts in NAD metabolism and biology that are reshaping the field. In addition, we will pose some open questions in NAD biology, including why NAD metabolism is so fast and dynamic in some tissues, how NAD and its precursors are transported to cells and organelles, and how NAD metabolism is integrated with inflammation and senescence. Resolving these questions will lead to significant advancements in the field.
Keywords: NAD pathway metabolites; NAD(+); aging; disease; humans; mitochondria; transport; vitamin B3.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests E.N.C. holds a patent on the use of CD38 inhibitors for metabolic diseases that is licensed by Elysium health. E.N.C. is a consultant for TeneoBio, Calico, Mitobridge, and Cytokinetics. E.N.C. is on the advisory board of Eolo Pharma. E.N.C. own stocks in TeneoBio. Research in the E.N.C. laboratory has been conducted in compliance with Mayo Clinic conflict of interest policies.
Figures
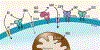
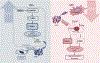
References
-
- Aljakna Khan A, Bararpour N, Gorka M, Joye T, Morel S, Montessuit CA, Grabherr S, Fracasso T, Augsburger M, Kwak BR, Thomas A, Sabatasso S. Detecting early myocardial ischemia in rat heart by MALDI imaging mass spectrometry. Sci Rep. 2021. March 4;11(1):5135. doi: 10.1038/s41598-021-84523-z. PMID: 33664384. - DOI - PMC - PubMed
-
- Andersen C, Maier E, Kemmer G, Blass J, Hilpert AK, Benz R, Reidl J. Porin OmpP2 of Haemophilus influenzae shows specificity for nicotinamide-derived nucleotide substrates. J Biol Chem. 2003; 278:24269–24276. - PubMed
-
- Bagcchi S. Nicotinamide yields impressive results in skin cancer. Lancet Oncol. 2015; 16: e591. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

