Regulation of tau internalization, degradation, and seeding by LRP1 reveals multiple pathways for tau catabolism
- PMID: 33930462
- PMCID: PMC8164048
- DOI: 10.1016/j.jbc.2021.100715
Regulation of tau internalization, degradation, and seeding by LRP1 reveals multiple pathways for tau catabolism
Abstract
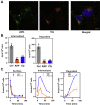
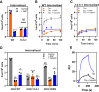
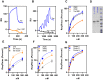
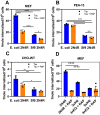
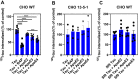
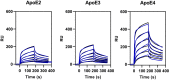
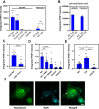
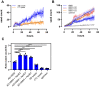
In Alzheimer's disease (AD), pathological forms of tau are transferred from cell to cell and "seed" aggregation of cytoplasmic tau. Phosphorylation of tau plays a key role in neurodegenerative tauopathies. In addition, apolipoprotein E (apoE), a major component of lipoproteins in the brain, is a genetic risk determinant for AD. The identification of the apoE receptor, low-density lipoprotein receptor-related protein 1 (LRP1), as an endocytic receptor for tau raises several questions about the role of LRP1 in tauopathies: is internalized tau, like other LRP1 ligands, delivered to lysosomes for degradation, and does LRP1 internalize pathological tau leading to cytosolic seeding? We found that LRP1 rapidly internalizes 125I-labeled tau, which is then efficiently degraded in lysosomal compartments. Surface plasmon resonance experiments confirm high affinity binding of tau and the tau microtubule-binding domain to LRP1. Interestingly, phosphorylated forms of recombinant tau bind weakly to LRP1 and are less efficiently internalized by LRP1. LRP1-mediated uptake of tau is inhibited by apoE, with the apoE4 isoform being the most potent inhibitor, likely because of its higher affinity for LRP1. Employing post-translationally-modified tau derived from brain lysates of human AD brain tissue, we found that LRP1-expressing cells, but not LRP1-deficient cells, promote cytosolic tau seeding in a process enhanced by apoE. These studies identify LRP1 as an endocytic receptor that binds and processes monomeric forms of tau leading to its degradation and promotes seeding by pathological forms of tau. The balance of these processes may be fundamental to the spread of neuropathology across the brain in AD.
Keywords: Alzheimer' s disease; Apolipoprotien E; LRP1; catabolism; lipoprotein receptors; neurofibrillary tangles; neurons; surface plasmon resonance; tau; tau spreading.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures

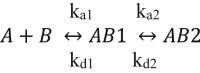
Similar articles
-
SORL1 is a receptor for tau that promotes tau seeding.J Biol Chem. 2024 Jun;300(6):107313. doi: 10.1016/j.jbc.2024.107313. Epub 2024 Apr 23. J Biol Chem. 2024. PMID: 38657864 Free PMC article.
-
LRP1 at the crossroads of Parkinson's and Alzheimer's: Divergent roles in α-synuclein and amyloid pathology.Eur J Pharmacol. 2025 Sep 5;1002:177830. doi: 10.1016/j.ejphar.2025.177830. Epub 2025 Jun 6. Eur J Pharmacol. 2025. PMID: 40484331 Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
The LDL receptor-related protein 1 (LRP1) facilitates ACE2-mediated endocytosis of SARS-CoV2 spike protein-containing pseudovirions.J Biol Chem. 2025 Jun;301(6):110227. doi: 10.1016/j.jbc.2025.110227. Epub 2025 May 9. J Biol Chem. 2025. PMID: 40349772 Free PMC article.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
Cited by
-
Updates in Alzheimer's disease: from basic research to diagnosis and therapies.Transl Neurodegener. 2024 Sep 4;13(1):45. doi: 10.1186/s40035-024-00432-x. Transl Neurodegener. 2024. PMID: 39232848 Free PMC article. Review.
-
Punicalagin's Protective Effects on Parkinson's Progression in Socially Isolated and Socialized Rats: Insights into Multifaceted Pathway.Pharmaceutics. 2023 Oct 4;15(10):2420. doi: 10.3390/pharmaceutics15102420. Pharmaceutics. 2023. PMID: 37896179 Free PMC article.
-
Fibrillar tau alters cerebral endothelial cell metabolism, vascular inflammatory activation, and barrier function in vitro and in vivo.Alzheimers Dement. 2025 Mar;21(3):e70077. doi: 10.1002/alz.70077. Alzheimers Dement. 2025. PMID: 40110691 Free PMC article.
-
Cytosolic antibody receptor TRIM21 is required for effective tau immunotherapy in mouse models.Science. 2023 Mar 31;379(6639):1336-1341. doi: 10.1126/science.abn1366. Epub 2023 Mar 30. Science. 2023. PMID: 36996217 Free PMC article.
-
APOE in the bullseye of neurodegenerative diseases: impact of the APOE genotype in Alzheimer's disease pathology and brain diseases.Mol Neurodegener. 2022 Sep 24;17(1):62. doi: 10.1186/s13024-022-00566-4. Mol Neurodegener. 2022. PMID: 36153580 Free PMC article. Review.
References
-
- Hyman B.T., Van Hoesen G.W., Damasio A.R., Clifford L. Alzheimer’s disease: Cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. - PubMed
-
- Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. - PubMed
-
- Polydoro M., de Calignon A., Suárez-Calvet M., Sanchez L., Kay K.R., Nicholls S.B., Roe A.D., Pitstick R., Carlson G.A., Gómez-Isla T., Spires-Jones T.L., Hyman B.T. Reversal of neurofibrillary tangles and tau-associated phenotype in the rTgTauEC model of early Alzheimer’s disease. J. Neurosci. 2013;33:13300–13311. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

