Recent advances and challenges of RT-PCR tests for the diagnosis of COVID-19
- PMID: 33930607
- PMCID: PMC8045416
- DOI: 10.1016/j.prp.2021.153443
Recent advances and challenges of RT-PCR tests for the diagnosis of COVID-19
Abstract
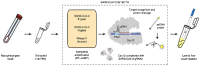
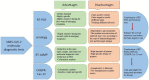
Since the outbreak of the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the control of virus spread has remained challenging given the pitfalls of the current diagnostic tests. Nevertheless, RNA amplification techniques have been the gold standard among other diagnostic methods for monitoring clinical samples for the presence of the virus. In the current paper, we review the shortcomings and strengths of RT-PCR (real-time polymerase chain reaction) techniques for diagnosis of coronavirus disease (COVID)-19. We address the repercussions of false-negative and false-positive rates encountered in the test, summarize approaches to improve the overall sensitivity of this method. We discuss the barriers to the widespread use of the RT-PCR test, and some technical advances, such as RT-LAMP (reverse-transcriptase-loop mediated isothermal amplification). We also address how other molecular techniques, such as immunodiagnostic tests can be used to avoid incorrect interpretation of RT-PCR tests.
Keywords: COVID-19; Diagnostic tests; Reverse transcription loop-mediated isothermal amplification; Reverse transcription polymerase chain reaction; SARS-CoV-2; Serologic tests.
Copyright © 2021 Elsevier GmbH. All rights reserved.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous