Pharmacist-Led Mobile Health Intervention and Transplant Medication Safety: A Randomized Controlled Clinical Trial
- PMID: 33931415
- PMCID: PMC8259471
- DOI: 10.2215/CJN.15911020
Pharmacist-Led Mobile Health Intervention and Transplant Medication Safety: A Randomized Controlled Clinical Trial
Abstract
Background and objectives: Medication safety events are predominant contributors to suboptimal graft outcomes in kidney transplant recipients. The goal of this study was to examine the efficacy of improving medication safety through a pharmacist-led, mobile health-based intervention.
Design, setting, participants, & measurements: This was a 12-month, single-center, prospective, parallel, two-arm, single-blind, randomized controlled trial. Adult kidney recipients 6-36 months post-transplant were eligible. Participants randomized to intervention received supplemental clinical pharmacist-led medication therapy monitoring and management via a mobile health-based application, integrated with risk-guided televisits and home-based BP and glucose monitoring. The application provided an accurate medication regimen, timely reminders, and side effect surveys. Both the control and intervention arms received usual care, including serial laboratory monitoring and regular clinic visits. The coprimary outcomes were to assess the incidence and severity of medication errors and adverse events.
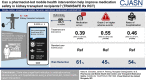
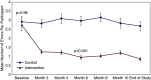
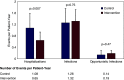
Results: In total, 136 kidney transplant recipients were included, 68 in each arm. The mean age was 51 years, 57% were male, and 64% were Black individuals. Participants receiving the intervention experienced a significant reduction in medication errors (61% reduction in the risk rate; incident risk ratio, 0.39; 95% confidence interval, 0.28 to 0.55; P<0.001) and a significantly lower incidence risk of Grade 3 or higher adverse events (incident risk ratio, 0.55, 95% confidence interval, 0.30 to 0.99; P=0.05). For the secondary outcome of hospitalizations, the intervention arm demonstrated significantly lower rates of hospitalizations (incident risk ratio, 0.46; 95% confidence interval, 0.27 to 0.77; P=0.005).
Conclusions: We demonstrated a significant reduction in medication errors, adverse events, and hospitalizations using a pharmacist-led, mobile health-based intervention.
Keywords: drug interactions; hospitalization; kidney transplantation; pharmacists.
Copyright © 2021 by the American Society of Nephrology.
Figures


Comment in
-
Twenty-First Century Solutions to Increase Medication Optimization and Safety in Kidney Transplant Patients.Clin J Am Soc Nephrol. 2021 May 8;16(5):679-681. doi: 10.2215/CJN.04160321. Epub 2021 Apr 30. Clin J Am Soc Nephrol. 2021. PMID: 33931416 Free PMC article. No abstract available.
References
-
- Taber DJ, Pilch NA, McGillicuddy JW, Mardis C, Treiber F, Fleming JN: Using informatics and mobile health to improve medication safety monitoring in kidney transplant recipients. Am J Health Syst Pharm 76: 1143–1149, 2019. - PubMed
-
- Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Castro S, Foutz J, Wainright JL, Snyder JJ, Kasiske BL, Israni AK: OPTN/SRTR 2018 annual data report: Kidney. Am J Transplant 20[Suppl s1]: 20–130, 2020. - PubMed
-
- Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B: Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant 4: 378–383, 2004. - PubMed
-
- Meier-Kriesche HU, Schold JD, Kaplan B: Long-term renal allograft survival: Have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am J Transplant 4: 1289–1295, 2004. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

