Emerging Roles for AKT Isoform Preference in Cancer Progression Pathways
- PMID: 33931488
- PMCID: PMC8349788
- DOI: 10.1158/1541-7786.MCR-20-1066
Emerging Roles for AKT Isoform Preference in Cancer Progression Pathways
Abstract
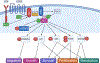
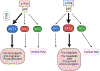
The phosphoinositol-3 kinase (PI3K)-AKT pathway is one of the most mutated in human cancers, predominantly associated with the loss of the signaling antagonist, PTEN, and to lesser extents, with gain-of-function mutations in PIK3CA (encoding PI3K-p110α) and AKT1. In addition, most oncogenic driver pathways activate PI3K/AKT signaling. Nonetheless, drugs targeting PI3K or AKT have fared poorly against solid tumors in clinical trials as monotherapies, yet some have shown efficacy when combined with inhibitors of other oncogenic drivers, such as receptor tyrosine kinases or nuclear hormone receptors. There is growing evidence that AKT isoforms, AKT1, AKT2, and AKT3, have different, often distinct roles in either promoting or suppressing specific parameters of oncogenic progression, yet few if any isoform-preferred substrates have been characterized. This review will describe recent data showing that the differential activation of AKT isoforms is mediated by complex interplays between PTEN, PI3K isoforms and upstream tyrosine kinases, and that the efficacy of PI3K/AKT inhibitors will likely depend on the successful targeting of specific AKT isoforms and their preferred pathways.
©2021 American Association for Cancer Research.
Conflict of interest statement
Figures
Similar articles
-
Molecular Insights of Pathways Resulting from Two Common PIK3CA Mutations in Breast Cancer.Cancer Res. 2016 Jul 1;76(13):3989-4001. doi: 10.1158/0008-5472.CAN-15-3174. Epub 2016 Apr 25. Cancer Res. 2016. PMID: 27197157
-
Role of PI3K and AKT specific isoforms in ovarian cancer cell migration, invasion and proliferation through the p70S6K1 pathway.Cell Signal. 2006 Dec;18(12):2262-71. doi: 10.1016/j.cellsig.2006.05.019. Epub 2006 Jun 2. Cell Signal. 2006. PMID: 16839745
-
Targeting AKT/PKB to improve treatment outcomes for solid tumors.Mutat Res. 2020 Jan-Apr;819-820:111690. doi: 10.1016/j.mrfmmm.2020.111690. Epub 2020 Feb 20. Mutat Res. 2020. PMID: 32120136 Free PMC article. Review.
-
Genetic alterations in the phosphatidylinositol-3 kinase/Akt pathway in thyroid cancer.Thyroid. 2010 Jul;20(7):697-706. doi: 10.1089/thy.2010.1646. Thyroid. 2010. PMID: 20578891 Free PMC article. Review.
-
Mutational and immunohistochemical study of the PI3K/Akt pathway in papillary thyroid carcinoma in Greece.Endocr Pathol. 2010 Jun;21(2):90-100. doi: 10.1007/s12022-010-9112-0. Endocr Pathol. 2010. PMID: 20186503
Cited by
-
AKT Regulation of ORAI1-Mediated Calcium Influx in Breast Cancer Cells.Cancers (Basel). 2022 Sep 30;14(19):4794. doi: 10.3390/cancers14194794. Cancers (Basel). 2022. PMID: 36230716 Free PMC article.
-
Addressing the Reciprocal Crosstalk between the AR and the PI3K/AKT/mTOR Signaling Pathways for Prostate Cancer Treatment.Int J Mol Sci. 2023 Jan 24;24(3):2289. doi: 10.3390/ijms24032289. Int J Mol Sci. 2023. PMID: 36768610 Free PMC article. Review.
-
The roles of AKT isoforms in decidualization and embryo implantation using a Progesterone Receptor-Cre mouse model†.Biol Reprod. 2025 Jun 15;112(6):1134-1147. doi: 10.1093/biolre/ioaf062. Biol Reprod. 2025. PMID: 40143411 Free PMC article.
-
The Role of PI3K/AKT/mTOR Signaling in Tumor Radioresistance and Advances in Inhibitor Research.Int J Mol Sci. 2025 Jul 17;26(14):6853. doi: 10.3390/ijms26146853. Int J Mol Sci. 2025. PMID: 40725100 Free PMC article. Review.
-
Predictive biomarkers for metachronous gastric cancer development after endoscopic resection of early gastric cancer.Cancer Med. 2024 Aug;13(16):e70104. doi: 10.1002/cam4.70104. Cancer Med. 2024. PMID: 39171503 Free PMC article.
References
-
- Nakatani K, Sakaue H, Thompson DA, Weigel RJ, Roth RA. Identification of a human Akt3 (Protein kinase B γ) which contains the regulatory serine phosphorylation site. Biochem Biophys Res Commun 1999;257:906–10. - PubMed
-
- Franke TF, Yang S-I, Chan TO, Datta K, Kazlauskas A, Morrison DK, et al. The Protein Kinase Encoded by the Akt Proto-Oncogene Is a Target of the PDGF-Activated Phosphatidylinositol 3-Kinase. Cell 1995;81:727–36. - PubMed
-
- Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science 1997;275:665–8. - PubMed
-
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275:1943–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

