Physiology of Cultured Human Microglia Maintained in a Defined Culture Medium
- PMID: 33931497
- PMCID: PMC9190148
- DOI: 10.4049/immunohorizons.2000101
Physiology of Cultured Human Microglia Maintained in a Defined Culture Medium
Abstract
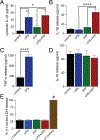
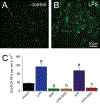
Microglia are the primary immune cell of the CNS, comprising 5-20% of the ∼60 billion neuroglia in the human brain. In the developing and adult CNS, they preferentially target active neurons to guide synapse maturation and remodeling. At the same time, they are the first line of defense against bacterial, fungal, and viral CNS infections. Although an extensive literature details their roles in rodents, less is known about how they function in humans because of the difficulty in obtaining tissue samples and the understandable inability to extensively study human microglia in situ. In this study, we use recent advances in the study of brain microenvironments to establish cultures of primary human microglia in a serum-free medium. Postsurgical samples of human brain were enzymatically and mechanically dissociated into single cells, and microglia were isolated at high purity by positive selection using CD11b Ab-coated microbeads. The CD11b+ cells were plated on poly-l-lysine-coated surfaces and bathed in serum-free DMEM/F12 supplemented with three essential components (TGF-β, IL-34, and cholesterol). Under these conditions, microglia assumed a ramified morphology, showed limited proliferation, actively surveyed their surroundings, and phagocytosed bacterial microparticles. In the presence of LPS, they assumed a more compact shape and began production of proinflammatory cytokines and reactive oxygen species. LPS on its own triggered release of TNF-α, whereas release of IL-1β required costimulation by ATP. Thus, human microglia maintained in a defined medium replicate many of the characteristics expected of native cells in the brain and provide an accessible preparation for investigations of human microglial physiology, pharmacology, and pathophysiology.
Copyright © 2021 The Authors.
Figures






References
-
- Wolf SA, Boddeke HW, and Kettenmann H. 2017. Microglia in Physiology and Disease. Annu Rev Physiol 79: 619–643. - PubMed
-
- Nimmerjahn A, Kirchhoff F, and Helmchen F. 2005. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308: 1314–1318. - PubMed
-
- Hanisch UK, and Kettenmann H. 2007. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10: 1387–1394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

