Rules of formation of H-C-N-O compounds at high pressure and the fates of planetary ices
- PMID: 33931549
- PMCID: PMC8126778
- DOI: 10.1073/pnas.2026360118
Rules of formation of H-C-N-O compounds at high pressure and the fates of planetary ices
Abstract
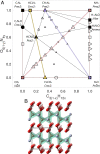
The solar system's outer planets, and many of their moons, are dominated by matter from the H-C-N-O chemical space, based on solar system abundances of hydrogen and the planetary ices [Formula: see text]O, [Formula: see text], and [Formula: see text] In the planetary interiors, these ices will experience extreme pressure conditions, around 5 Mbar at the Neptune mantle-core boundary, and it is expected that they undergo phase transitions, decompose, and form entirely new compounds. While temperature will dictate the formation of compounds, ground-state density functional theory allows us to probe the chemical effects resulting from pressure alone. These structural developments in turn determine the planets' interior structures, thermal evolution, and magnetic field generation, among others. Despite its importance, the H-C-N-O system has not been surveyed systematically to explore which compounds emerge at high-pressure conditions, and what governs their stability. Here, we report on and analyze an unbiased crystal structure search among H-C-N-O compounds between 1 and 5 Mbar. We demonstrate that simple chemical rules drive stability in this composition space, which explains why the simplest possible quaternary mixture HCNO-isoelectronic to diamond-emerges as a stable compound and discuss dominant decomposition products of planetary ice mixtures.
Keywords: H–C–N–O chemistry; high pressure; planetary ices; structure search.
Conflict of interest statement
The authors declare no competing interest.
Figures





References
-
- Dong X., et al. , A stable compound of helium and sodium at high pressure. Nat. Chem. 9, 440–445 (2017). - PubMed
-
- Duan D., et al. , Pressure-induced decomposition of solid hydrogen sulfide. Phys. Rev. B Condens. Matter Mater. Phys. 91, 180502 (2015).
-
- Drozdov A. P., Eremets M. I., Troyan I. A., Ksenofontov V., Shylin S. I., Conventional superconductivity at 203 kelvin at high pressures in the sulfur hydride system. Nature 525, 73–76 (2015). - PubMed
-
- Peng F., et al. , Hydrogen clathrate structures in rare Earth hydrides at high pressures: Possible route to room-temperature superconductivity. Phys. Rev. Lett. 119, 107001 (2017). - PubMed
-
- Geballe Z. M., et al. , Synthesis and stability of lanthanum superhydrides. Angew. Chem. Int. Ed. 57, 688–692 (2018). - PubMed
Publication types
LinkOut - more resources
Full Text Sources

