CO2 supply modulates lipid remodelling, photosynthetic and respiratory activities in Chlorella species
- PMID: 33931891
- PMCID: PMC8453743
- DOI: 10.1111/pce.14074
CO2 supply modulates lipid remodelling, photosynthetic and respiratory activities in Chlorella species
Abstract
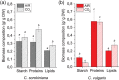
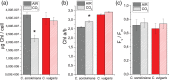
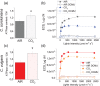
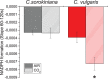
Microalgae represent a potential solution to reduce CO2 emission exploiting their photosynthetic activity. Here, the physiologic and metabolic responses at the base of CO2 assimilation were investigated in conditions of high or low CO2 availability in two of the most promising algae species for industrial cultivation, Chlorella sorokiniana and Chlorella vulgaris. In both species, high CO2 availability increased biomass accumulation with specific increase of triacylglycerols in C. vulgaris and polar lipids and proteins in C. sorokiniana. Moreover, high CO2 availability caused only in C. vulgaris a reduced NAD(P)H/NADP+ ratio and reduced mitochondrial respiration, suggesting a CO2 dependent increase of reducing power consumption in the chloroplast, which in turn influences the redox state of the mitochondria. Several rearrangements of the photosynthetic machinery were observed in both species, differing from those described for the model organism Chlamydomonas reinhardtii, where adaptation to carbon availability is mainly controlled by the translational repressor NAB1. NAB1 homologous protein could be identified only in C. vulgaris but lacked the regulation mechanisms previously described in C. reinhardtii. Acclimation strategies to cope with a fluctuating inorganic carbon supply are thus diverse among green microalgae, and these results suggest new biotechnological strategies to boost CO2 fixation.
Keywords: Chlorophyta; carbon assimilation; chlorella; lipids; microalgae; photosynthesis; respiration; triacylglycerols.
© 2021 The Authors. Plant, Cell & Environment published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

