Epitope mapping of novel monoclonal antibodies to human angiotensin I-converting enzyme
- PMID: 33931897
- PMCID: PMC8284578
- DOI: 10.1002/pro.4091
Epitope mapping of novel monoclonal antibodies to human angiotensin I-converting enzyme
Abstract
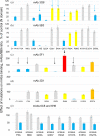
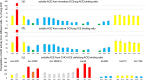
Angiotensin I-converting enzyme (ACE, CD143) plays a crucial role in blood pressure regulation, vascular remodeling, and immunity. A wide spectrum of mAbs to different epitopes on the N and C domains of human ACE have been generated and used to study different aspects of ACE biology, including establishing a novel approach-conformational fingerprinting. Here we characterized a novel set of 14 mAbs, developed against human seminal fluid ACE. The epitopes for these novel mAbs were defined using recombinant ACE constructs with truncated N and C domains, species cross-reactivity, ACE mutagenesis, and competition with the previously mapped anti-ACE mAbs. Nine mAbs recognized regions on the N domain, and 5 mAbs-on the C domain of ACE. The epitopes for most of these novel mAbs partially overlap with epitopes mapped onto ACE by the previously generated mAbs, whereas mAb 8H1 recognized yet unmapped region on the C domain where three ACE mutations associated with Alzheimer's disease are localized and is a marker for ACE mutation T877M. mAb 2H4 could be considered as a specific marker for ACE in dendritic cells. This novel set of mAbs can identify even subtle changes in human ACE conformation caused by tissue-specific glycosylation of ACE or mutations, and can detect human somatic and testicular ACE in biological fluids and tissues. Furthermore, the high reactivity of these novel mAbs provides an opportunity to study changes in the pattern of ACE expression or glycosylation in different tissues, cells, and diseases, such as sarcoidosis and Alzheimer's disease.
Keywords: ACE mutations; Alzheimer's disease; CD143; angiotensin I-converting enzyme; conformational fingerprinting; dendritic cells; glycosylation; prostate; sarcoidosis.
© 2021 The Protein Society.
Conflict of interest statement
Dr. Garcia is Founder and CEO of Aqualung Therapeutics Corp. (Tucson, AZ).
Figures




References
-
- Sturrock ED, Anthony CS, Danilov SM. Peptidyl‐dipeptidase a/angiotensin I‐converting enzyme. In: Rawlings ND, Salvesen G, editors. Handbook of Proteolytic Enzymes. 3rd ed. Oxford: Academic Press, 2012; p. 480–494.
-
- Jokubaitis VJ, Sinka L, Driessen R, et al. Angiotensin‐converting enzyme (CD143) marks hematopoietic stem cells in human embryonic, fetal and adult hematopoietic tissues. Blood. 2008;111:4055–4063. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

