Regulation of capillary hemodynamics by KATP channels in resting skeletal muscle
- PMID: 33932103
- PMCID: PMC8087980
- DOI: 10.14814/phy2.14803
Regulation of capillary hemodynamics by KATP channels in resting skeletal muscle
Abstract
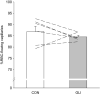
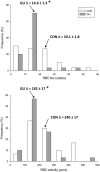
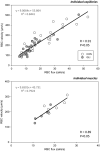
ATP-sensitive K+ channels (KATP ) have been implicated in the regulation of resting vascular smooth muscle membrane potential and tone. However, whether KATP channels modulate skeletal muscle microvascular hemodynamics at the capillary level (the primary site for blood-myocyte O2 exchange) remains unknown. We tested the hypothesis that KATP channel inhibition would reduce the proportion of capillaries supporting continuous red blood cell (RBC) flow and impair RBC hemodynamics and distribution in perfused capillaries within resting skeletal muscle. RBC flux (fRBC ), velocity (VRBC ), and capillary tube hematocrit (Hctcap ) were assessed via intravital microscopy of the rat spinotrapezius muscle (n = 6) under control (CON) and glibenclamide (GLI; KATP channel antagonist; 10 µM) superfusion conditions. There were no differences in mean arterial pressure (CON:120 ± 5, GLI:124 ± 5 mmHg; p > 0.05) or heart rate (CON:322 ± 32, GLI:337 ± 33 beats/min; p > 0.05) between conditions. The %RBC-flowing capillaries were not altered between conditions (CON:87 ± 2, GLI:85 ± 1%; p > 0.05). In RBC-perfused capillaries, GLI reduced fRBC (CON:20.1 ± 1.8, GLI:14.6 ± 1.3 cells/s; p < 0.05) and VRBC (CON:240 ± 17, GLI:182 ± 17 µm/s; p < 0.05) but not Hctcap (CON:0.26 ± 0.01, GLI:0.26 ± 0.01; p > 0.05). The absence of GLI effects on the %RBC-flowing capillaries and Hctcap indicates preserved muscle O2 diffusing capacity (DO2 m). In contrast, GLI lowered both fRBC and VRBC thus impairing perfusive microvascular O2 transport (Q̇m) and lengthening RBC capillary transit times, respectively. Given the interdependence between diffusive and perfusive O2 conductances (i.e., %O2 extraction∝DO2 m/Q̇m), such GLI alterations are expected to elevate muscle %O2 extraction to sustain a given metabolic rate. These results support that KATP channels regulate capillary hemodynamics and, therefore, microvascular gas exchange in resting skeletal muscle.
Keywords: ATP-sensitive K+ channel; blood flow; intravital microscopy; microcirculation; red blood cell.
© 2021 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

Similar articles
-
KATP channel inhibition-induced hyporemia in skeletal muscle: No evidence for pre-capillary sphincter action.Microvasc Res. 2025 Jul;160:104808. doi: 10.1016/j.mvr.2025.104808. Epub 2025 Mar 29. Microvasc Res. 2025. PMID: 40164381
-
Sexual dimorphism in vascular ATP-sensitive K+ channel function supporting interstitial via convective and/or diffusive O2 transport.J Physiol. 2021 Jul;599(13):3279-3293. doi: 10.1113/JP281120. Epub 2021 Jun 8. J Physiol. 2021. PMID: 34101850 Free PMC article.
-
Post-occlusive reactive hyperemia and skeletal muscle capillary hemodynamics.Microvasc Res. 2022 Mar;140:104283. doi: 10.1016/j.mvr.2021.104283. Epub 2021 Nov 22. Microvasc Res. 2022. PMID: 34822837 Free PMC article.
-
Dynamics of muscle microcirculatory and blood-myocyte O(2) flux during contractions.Acta Physiol (Oxf). 2011 Jul;202(3):293-310. doi: 10.1111/j.1748-1716.2010.02246.x. Epub 2011 Mar 1. Acta Physiol (Oxf). 2011. PMID: 21199399 Free PMC article. Review.
-
Skeletal muscle capillary function: contemporary observations and novel hypotheses.Exp Physiol. 2013 Dec;98(12):1645-58. doi: 10.1113/expphysiol.2013.073874. Epub 2013 Aug 30. Exp Physiol. 2013. PMID: 23995101 Free PMC article. Review.
Cited by
-
KATP channel inhibition-induced hyporemia in skeletal muscle: No evidence for pre-capillary sphincter action.Microvasc Res. 2025 Jul;160:104808. doi: 10.1016/j.mvr.2025.104808. Epub 2025 Mar 29. Microvasc Res. 2025. PMID: 40164381
-
Kir6.1 and SUR2B in Cantú syndrome.Am J Physiol Cell Physiol. 2022 Sep 1;323(3):C920-C935. doi: 10.1152/ajpcell.00154.2022. Epub 2022 Jul 25. Am J Physiol Cell Physiol. 2022. PMID: 35876283 Free PMC article. Review.
-
Capillary-Mitochondrial Oxygen Transport in Muscle: Paradigm Shifts.Function (Oxf). 2023 Mar 16;4(3):zqad013. doi: 10.1093/function/zqad013. eCollection 2023. Function (Oxf). 2023. PMID: 37168497 Free PMC article. Review.
-
Effects of pulmonary hypertension on microcirculatory hemodynamics in rat skeletal muscle.Microvasc Res. 2022 May;141:104334. doi: 10.1016/j.mvr.2022.104334. Epub 2022 Jan 30. Microvasc Res. 2022. PMID: 35104507 Free PMC article.
-
Sexual dimorphism in vascular ATP-sensitive K+ channel function supporting interstitial via convective and/or diffusive O2 transport.J Physiol. 2021 Jul;599(13):3279-3293. doi: 10.1113/JP281120. Epub 2021 Jun 8. J Physiol. 2021. PMID: 34101850 Free PMC article.
References
-
- Altman, P. , & Dittmer, D. (1974). Biology data book, 2nd ed. Federation of American Societies for Experimental Biology.
-
- Bailey, J. K. , Kindig, C. A. , Behnke, B. J. , Musch, T. I. , Schmid‐Schoenbein, G. W. , & Poole, D. C. (2000). Spinotrapezius muscle microcirculatory function: Effects of surgical exteriorization. American Journal of Physiology. Heart and Circulatory Physiology, 279, H3131–3137. 10.1152/ajpheart.2000.279.6.H3131. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

