Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors
- PMID: 33932508
- PMCID: PMC8080507
- DOI: 10.1016/j.annonc.2021.04.019
Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors
Conflict of interest statement
Disclosure The authors have declared no conflicts of interest.
Figures
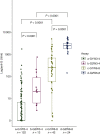
Comment in
-
High levels of anti-SARS-CoV-2 IgG antibodies in previously infected patients with cancer after a single dose of BNT 162b2 vaccine.Eur J Cancer. 2021 Sep;154:4-6. doi: 10.1016/j.ejca.2021.05.036. Epub 2021 Jun 11. Eur J Cancer. 2021. PMID: 34217909 Free PMC article. No abstract available.
-
Reduced SARS-CoV-2 infection and death after two doses of COVID-19 vaccines in a series of 1503 cancer patients.Ann Oncol. 2021 Nov;32(11):1443-1444. doi: 10.1016/j.annonc.2021.07.012. Epub 2021 Jul 30. Ann Oncol. 2021. PMID: 34333128 Free PMC article. No abstract available.
References
-
- Meerveld-Eggink A., de Weerdt O., van der Velden A.M.T. Response to influenza virus vaccination during chemotherapy in patients with breast cancer. Ann Oncol. 2011;22(9):2031–2035. - PubMed
-
- Rousseau B., Loulergue P., Mir O. Immunogenicity and safety of the influenza A H1N1v 2009 vaccine in cancer patients treated with cytotoxic chemotherapy and/or targeted therapy: the VACANCE study. Ann Oncol. 2012;23(2):450–457. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

