Nicotinamide provides neuroprotection in glaucoma by protecting against mitochondrial and metabolic dysfunction
- PMID: 33932867
- PMCID: PMC8103000
- DOI: 10.1016/j.redox.2021.101988
Nicotinamide provides neuroprotection in glaucoma by protecting against mitochondrial and metabolic dysfunction
Abstract
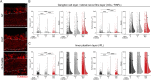
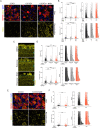
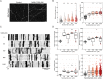
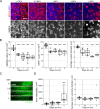
Nicotinamide adenine dinucleotide (NAD) is a REDOX cofactor and metabolite essential for neuronal survival. Glaucoma is a common neurodegenerative disease in which neuronal levels of NAD decline. We assess the effects of nicotinamide (a precursor to NAD) on retinal ganglion cells (the affected neuron in glaucoma) in normal physiological conditions and across a range of glaucoma relevant insults including mitochondrial stress and axon degenerative insults. We demonstrate retinal ganglion cell somal, axonal, and dendritic neuroprotection by nicotinamide in rodent models which represent isolated ocular hypertensive, axon degenerative, and mitochondrial degenerative insults. We performed metabolomics enriched for small molecular weight metabolites for the retina, optic nerve, and superior colliculus which demonstrates that ocular hypertension induces widespread metabolic disruption, including consistent changes to α-ketoglutaric acid, creatine/creatinine, homocysteine, and glycerophosphocholine. This metabolic disruption is prevented by nicotinamide. Nicotinamide provides further neuroprotective effects by increasing oxidative phosphorylation, buffering and preventing metabolic stress, and increasing mitochondrial size and motility whilst simultaneously dampening action potential firing frequency. These data support continued determination of the utility of long-term nicotinamide treatment as a neuroprotective therapy for human glaucoma.
Keywords: Glaucoma; Metabolism; Metabolomics; Mitochondria; Nicotinamide; Retina; Retinal ganglion cell.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures














References
-
- Tham Y.C., Li X., Wong T.Y., Quigley H.A., Aung T., Cheng C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. - PubMed
-
- Naik S., Pandey A., Lewis S.A., Rao B.S.S., Mutalik S. Neuroprotection: a versatile approach to combat glaucoma. Eur. J. Pharmacol. 2020;881:173208. - PubMed
-
- Peters D., Bengtsson B., Heijl A. Factors associated with lifetime risk of open-angle glaucoma blindness. Acta Ophthalmol. 2014;92(5):421–425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

