Glutaredoxin: Discovery, redox defense and much more
- PMID: 33932870
- PMCID: PMC8102999
- DOI: 10.1016/j.redox.2021.101975
Glutaredoxin: Discovery, redox defense and much more
Abstract
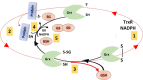
Glutaredoxin, Grx, is a small protein containing an active site cysteine pair and was discovered in 1976 by Arne Holmgren. The Grx system, comprised of Grx, glutathione, glutathione reductase, and NADPH, was first described as an electron donor for Ribonucleotide Reductase but, from the first discovery in E.coli, the Grx family has impressively grown, particularly in the last two decades. Several isoforms have been described in different organisms (from bacteria to humans) and with different functions. The unique characteristic of Grxs is their ability to catalyse glutathione-dependent redox regulation via glutathionylation, the conjugation of glutathione to a substrate, and its reverse reaction, deglutathionylation. Grxs have also recently been enrolled in iron sulphur cluster formation. These functions have been implied in various physiological and pathological conditions, from immune defense to neurodegeneration and cancer development thus making Grx a possible drug target. This review aims to give an overview on Grxs, starting by a phylogenetic analysis of vertebrate Grxs, followed by an analysis of the mechanisms of action, the specific characteristics of the different human isoforms and a discussion on aspects related to human physiology and diseases.
Keywords: Deglutathionylation; Glutaredoxin; Glutathionylation; Grxs phylogenetics; Iron homeostasis; Redox regulation.
Copyright © 2021 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors report no conflict of interest.
Figures









References
-
- Laurent T.C., Moore E.C., Reichard P. Enzymatic Synthesis of Deoxyribonucleotides. Iv. Isolation and and characterization of thioredoxin, the hydrogen donor from Escherichia coli b. J. Biol. Chem. 1964;239:3436–3444. http://www.ncbi.nlm.nih.gov/pubmed/14245400 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

