SARS-CoV-2 seroprevalence, and IgG concentration and pseudovirus neutralising antibody titres after infection, compared by HIV status: a matched case-control observational study
- PMID: 33933189
- PMCID: PMC8084354
- DOI: 10.1016/S2352-3018(21)00072-2
SARS-CoV-2 seroprevalence, and IgG concentration and pseudovirus neutralising antibody titres after infection, compared by HIV status: a matched case-control observational study
Abstract
Background: Most cohorts show similar or lower COVID-19 incidence among people living with HIV compared with the general population. However, incidence might be affected by lower testing rates among vulnerable populations. We aimed to compare SARS-CoV-2 IgG seroprevalence, disease severity, and neutralising antibody activity after infection among people with and without HIV receiving care in a county hospital system over a 3-month period.
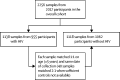
Methods: In this matched case-control observational study, remnant serum samples were collected between Aug 1 and Oct 31, 2020, from all people living with HIV who underwent routine outpatient laboratory testing in a municipal health-care system (San Francisco General Hospital, CA, USA). Samples from people living with HIV were date of collection-matched (same day) and age-matched (±5 years) to samples from randomly selected adults (aged 18 years or older) without HIV receiving care for chronic conditions at the same hospital. We compared seroprevalence by HIV status via mixed-effects logistic regression models, accounting for the matched structure of the data (random effects for the matched group), adjusting for age, sex, race or ethnicity, and clinical factors (ie, history of cardiovascular or pulmonary disease, and type 2 diabetes). Severe COVID-19 was assessed in participants with past SARS-CoV-2 (IgG or PCR) infection by chart review and compared with multivariable mixed-effects logistic regression, adjusting for age and sex. SARS-CoV-2 IgG, neutralising antibody titres, and antibody avidity were measured in serum of participants with previous positive PCR tests and compared with multivariable mixed-effects models, adjusting for age, sex, and time since PCR-confirmed SARS-CoV-2 infection.
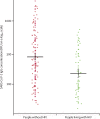
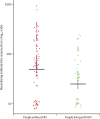
Findings: 1138 samples from 955 people living with HIV and 1118 samples from 1062 people without HIV were tested. SARS-CoV-2 IgG seroprevalence was 3·7% (95% CI 2·4 to 5·0) among people with HIV compared with 7·4% (5·7 to 9·2) among people without HIV (adjusted odds ratio 0·50, 95% CI 0·30 to 0·83). Among 31 people with HIV and 70 people without HIV who had evidence of past infection, the odds of severe COVID-19 were 5·52 (95% CI 1·01 to 64·48) times higher among people living with HIV. Adjusting for time since PCR-confirmed infection, SARS-CoV-2 IgG concentrations were lower (percentage change -53%, 95% CI -4 to -76), pseudovirus neutralising antibody titres were lower (-67%, -25 to -86), and avidity was similar (7%, -73 to 87) among people living with HIV compared with those without HIV.
Interpretation: Although fewer infections were detected by SARS-CoV-2 IgG testing among people living with HIV than among those without HIV, people with HIV had more cases of severe COVID-19. Among people living with HIV with past SARS-CoV-2 infection, lower IgG concentrations and pseudovirus neutralising antibody titres might reflect a diminished serological response to infection, and the similar avidity could be driven by similar time since infection.
Funding: US National Institute of Allergy and Infectious Diseases, US National Institutes of Health.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests MAS, DVG, TJH, MG, and LBB report funding from the US National Institutes of Health during conduct of the study. DVG reports personal fees from Gilead Sciences outside the submitted work. All other authors declare no competing interests.
Figures

Comment in
-
SARS-CoV-2 immunity and HIV infection: total recall?Lancet HIV. 2021 Jun;8(6):e312-e313. doi: 10.1016/S2352-3018(21)00097-7. Epub 2021 Apr 29. Lancet HIV. 2021. PMID: 33933190 Free PMC article. No abstract available.
Similar articles
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Sero-prevalence of SARS-CoV-2 antibodies in Ethiopia: Results of the National Population Based Survey, 2021.PLoS One. 2025 May 6;20(5):e0313791. doi: 10.1371/journal.pone.0313791. eCollection 2025. PLoS One. 2025. PMID: 40327714 Free PMC article.
-
Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. doi: 10.1002/14651858.CD013705.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866452 Free PMC article.
-
The effect of early COVID-19 treatment with convalescent plasma on antibody responses to SARS-CoV-2.Microbiol Spectr. 2025 Jul;13(7):e0300624. doi: 10.1128/spectrum.03006-24. Epub 2025 Jun 9. Microbiol Spectr. 2025. PMID: 40488473 Free PMC article. Clinical Trial.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
Cited by
-
Immunogenicity and reactogenicity of SARS-CoV-2 vaccines in people living with HIV in the Netherlands: A nationwide prospective cohort study.PLoS Med. 2022 Oct 27;19(10):e1003979. doi: 10.1371/journal.pmed.1003979. eCollection 2022 Oct. PLoS Med. 2022. PMID: 36301821 Free PMC article.
-
Multicenter observational survey on psychosocial and behavioral impacts of COVID-19 in people living with HIV in Northern Vietnam.Sci Rep. 2023 Nov 21;13(1):20321. doi: 10.1038/s41598-023-47577-9. Sci Rep. 2023. PMID: 37989776 Free PMC article.
-
Household Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 From Adult Index Cases With and Without Human Immunodeficiency Virus in South Africa, 2020-2021: A Case-Ascertained, Prospective, Observational Household Transmission Study.Clin Infect Dis. 2023 Feb 8;76(3):e71-e81. doi: 10.1093/cid/ciac640. Clin Infect Dis. 2023. PMID: 35925613 Free PMC article.
-
Long COVID in people living with HIV.Curr Opin HIV AIDS. 2023 May 1;18(3):126-134. doi: 10.1097/COH.0000000000000789. Epub 2023 Mar 20. Curr Opin HIV AIDS. 2023. PMID: 37144614 Free PMC article. Review.
-
SARS-CoV-2 seroprevalence among people living with HIV in the German HIV-1 Seroconverter Cohort, 2020-2022.BMC Infect Dis. 2024 Nov 1;24(1):1228. doi: 10.1186/s12879-024-10119-3. BMC Infect Dis. 2024. PMID: 39487409 Free PMC article.
References
-
- Park LS, Rentsch CT, Sigel K, et al. COVID-19 in the largest US HIV cohort. AIDS 2020: 23rd International AIDS Conference Virtual; July 6–10, 2020 (abstr LBPEC23).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

