Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial
- PMID: 33933206
- PMCID: PMC8084355
- DOI: 10.1016/S0140-6736(21)00676-0
Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial
Abstract
Background: In this study, we aimed to evaluate the effects of tocilizumab in adult patients admitted to hospital with COVID-19 with both hypoxia and systemic inflammation.
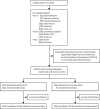
Methods: This randomised, controlled, open-label, platform trial (Randomised Evaluation of COVID-19 Therapy [RECOVERY]), is assessing several possible treatments in patients hospitalised with COVID-19 in the UK. Those trial participants with hypoxia (oxygen saturation <92% on air or requiring oxygen therapy) and evidence of systemic inflammation (C-reactive protein ≥75 mg/L) were eligible for random assignment in a 1:1 ratio to usual standard of care alone versus usual standard of care plus tocilizumab at a dose of 400 mg-800 mg (depending on weight) given intravenously. A second dose could be given 12-24 h later if the patient's condition had not improved. The primary outcome was 28-day mortality, assessed in the intention-to-treat population. The trial is registered with ISRCTN (50189673) and ClinicalTrials.gov (NCT04381936).
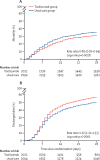
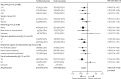
Findings: Between April 23, 2020, and Jan 24, 2021, 4116 adults of 21 550 patients enrolled into the RECOVERY trial were included in the assessment of tocilizumab, including 3385 (82%) patients receiving systemic corticosteroids. Overall, 621 (31%) of the 2022 patients allocated tocilizumab and 729 (35%) of the 2094 patients allocated to usual care died within 28 days (rate ratio 0·85; 95% CI 0·76-0·94; p=0·0028). Consistent results were seen in all prespecified subgroups of patients, including those receiving systemic corticosteroids. Patients allocated to tocilizumab were more likely to be discharged from hospital within 28 days (57% vs 50%; rate ratio 1·22; 1·12-1·33; p<0·0001). Among those not receiving invasive mechanical ventilation at baseline, patients allocated tocilizumab were less likely to reach the composite endpoint of invasive mechanical ventilation or death (35% vs 42%; risk ratio 0·84; 95% CI 0·77-0·92; p<0·0001).
Interpretation: In hospitalised COVID-19 patients with hypoxia and systemic inflammation, tocilizumab improved survival and other clinical outcomes. These benefits were seen regardless of the amount of respiratory support and were additional to the benefits of systemic corticosteroids.
Funding: UK Research and Innovation (Medical Research Council) and National Institute of Health Research.
Copyright © 2021 Published by Elsevier Ltd. All rights reserved. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests The authors have no conflict of interest or financial relationships relevant to the submitted work to disclose. No form of payment was given to anyone to produce the manuscript. All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. The Nuffield Department of Population Health at the University of Oxford has a staff policy of not accepting honoraria or consultancy fees directly or indirectly from industry.
Figures

Comment in
-
Tocilizumab in COVID-19: some clarity amid controversy.Lancet. 2021 May 1;397(10285):1599-1601. doi: 10.1016/S0140-6736(21)00712-1. Lancet. 2021. PMID: 33933194 Free PMC article. No abstract available.
-
Tocilizumab in COVID-19 therapy: who benefits, and how?Lancet. 2021 Jul 24;398(10297):299-300. doi: 10.1016/S0140-6736(21)01427-6. Lancet. 2021. PMID: 34303430 Free PMC article. No abstract available.
-
Tocilizumab in COVID-19 therapy: who benefits, and how?Lancet. 2021 Jul 24;398(10297):299. doi: 10.1016/S0140-6736(21)01380-5. Lancet. 2021. PMID: 34303431 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- 16896/CRUK_/Cancer Research UK/United Kingdom
- MR/S001751/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_00002/14/MRC_/Medical Research Council/United Kingdom
- MC_U137686861/MRC_/Medical Research Council/United Kingdom
- MC_PC_20058/MRC_/Medical Research Council/United Kingdom
- G0701652/MRC_/Medical Research Council/United Kingdom
- MR/K025643/1/MRC_/Medical Research Council/United Kingdom
- 211153/Z/18/Z/WT_/Wellcome Trust/United Kingdom
- MC_U137686860/MRC_/Medical Research Council/United Kingdom
- MC_UU_12026/4/MRC_/Medical Research Council/United Kingdom
- CS/18/2/33719/BHF_/British Heart Foundation/United Kingdom
- MC_PC_20062/MRC_/Medical Research Council/United Kingdom
- MC_PC_19056/MRC_/Medical Research Council/United Kingdom
- P30 AG059307/AG/NIA NIH HHS/United States
- G108/613/MRC_/Medical Research Council/United Kingdom
- G1002605/MRC_/Medical Research Council/United Kingdom
- SP/12/2/29422/BHF_/British Heart Foundation/United Kingdom
- MR/T005114/1/MRC_/Medical Research Council/United Kingdom
- 25350/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

