Allergic response to medical products in patients with alpha-gal syndrome
- PMID: 33933257
- PMCID: PMC9673037
- DOI: 10.1016/j.jtcvs.2021.03.100
Allergic response to medical products in patients with alpha-gal syndrome
Abstract
Background: Galactose-α-1,3-galactose (alpha-gal) is a carbohydrate that is ubiquitously expressed in all mammals except for primates and humans. Patients can become sensitized to this antigen and develop alpha-gal syndrome (AGS), or a red meat allergy. Symptoms range from generalized gastroenteritis and malaise to anaphylaxis, and in endemic areas, the prevalence can be as high as 20%. Although AGS patients commonly avoid alpha-gal by avoiding meat, patients have also developed symptoms due to animal-derived medical products and devices. With the rise in transcatheter aortic valve replacement, we investigate the immunogenicity of common cardiac materials and valves.
Objective: To assess the in vitro immunoglobulin E response toward common medical products, including cardiac patch materials and bioprosthetic valves in patients with AGS.
Methods: Immunoblot and immunohistochemistry techniques were applied to assess immunoglobulin E reactivity to various mammalian derived tissues and medical products for patients with AGS.
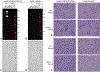
Results: AGS serum showed strong reactivity to all of the commercially available, nonhuman products tested, including various decellularized cardiac patch materials and bioprosthetic aortic valves. AGS serum did not react to tissues prepared using alpha-gal knockout pigs.
Conclusions: Despite commercial decellularization processes, alpha-gal continues to be present in animal-derived medical products, including bioprosthetic valves. Serum from patients with AGS demonstrates a strong affinity for these products in vitro. This may have serious potential implications for sensitized patients undergoing cardiac surgery, including early valve failure and accelerated coronary artery disease.
Keywords: allergy; alpha-gal; alpha-gal syndrome; bioprosthetic valve; chronic inflammation; coronary artery disease; early valve degradation.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Statement
The authors reported no conflicts of interest.
The
Figures










Comment in
-
Commentary: The decisive alpha-galactosyl hurdle after bioprosthesis implantation.J Thorac Cardiovasc Surg. 2022 Dec;164(6):e425-e426. doi: 10.1016/j.jtcvs.2021.04.008. Epub 2021 Apr 20. J Thorac Cardiovasc Surg. 2022. PMID: 33965221 No abstract available.
-
Commentary: Alpha-gal syndrome and cardiac implant durability.J Thorac Cardiovasc Surg. 2022 Dec;164(6):e426-e427. doi: 10.1016/j.jtcvs.2021.04.038. Epub 2021 Apr 20. J Thorac Cardiovasc Surg. 2022. PMID: 33994004 No abstract available.
References
-
- Platts-Mills TAE, Commins SP, Biedermann T, van Hage M, Levin M, Beck LA, et al. On the cause and consequences of IgE to galactose-alpha-1,3-galactose: a report from the National Institute of Allergy and Infectious Diseases Workshop on understanding IgE-mediated mammalian meat allergy. J Allergy Clin Immunol. 2020;145:1061–71. - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

