PIP2 regulation of TRPC5 channel activation and desensitization
- PMID: 33933453
- PMCID: PMC8191310
- DOI: 10.1016/j.jbc.2021.100726
PIP2 regulation of TRPC5 channel activation and desensitization
Abstract
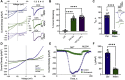
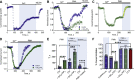
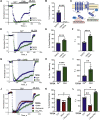
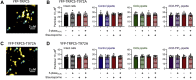
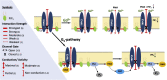
Transient receptor potential canonical type 5 (TRPC5) ion channels are expressed in the brain and kidney and have been identified as promising therapeutic targets whose selective inhibition can protect against diseases driven by a leaky kidney filter, such as focal segmental glomerular sclerosis. TRPC5 channels are activated not only by elevated levels of extracellular Ca2+or lanthanide ions but also by G protein (Gq/11) stimulation. Phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolysis by phospholipase C enzymes leads to PKC-mediated phosphorylation of TRPC5 channels and their subsequent desensitization. However, the roles of PIP2 in activation and maintenance of TRPC5 channel activity via its hydrolysis product diacyl glycerol (DAG), as well as the mechanism of desensitization of TRPC5 activity by DAG-stimulated PKC activity, remain unclear. Here, we designed experiments to distinguish between the processes underlying channel activation and inhibition. Employing whole-cell patch-clamp, we used an optogenetic tool to dephosphorylate PIP2 and assess channel-PIP2 interactions influenced by activators, such as DAG, or inhibitors, such as PKC phosphorylation. Using total internal reflection microscopy, we assessed channel cell surface density. We show that PIP2 controls both the PKC-mediated inhibition and the DAG- and lanthanide-mediated activation of TRPC5 currents via control of gating rather than channel cell surface density. These mechanistic insights promise to aid in the development of more selective and precise inhibitors to block TRPC5 channel activity and illuminate new opportunities for targeted therapies for a group of chronic kidney diseases for which there is currently a great unmet need.
Keywords: TRPC5 channels; diacyl glycerol (DAG); phosphatidylinositol 4,5-bisphosphate (PIP(2)); phosphatidylinositol signaling; phosphoinositide; transient receptor potential channels (TRP channels).
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest A. G. has a financial interest in Goldfinch Biopharma, which was reviewed and is managed by Brigham and Women’s Hospital, Mass General Brigham (MGB), and the Broad Institute of MIT and Harvard in accordance with their conflict-of-interest policies. All other authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

