Liver inflammation at the time of spinal cord injury enhances intraspinal pathology, liver injury, metabolic syndrome and locomotor deficits
- PMID: 33933462
- PMCID: PMC8784048
- DOI: 10.1016/j.expneurol.2021.113725
Liver inflammation at the time of spinal cord injury enhances intraspinal pathology, liver injury, metabolic syndrome and locomotor deficits
Abstract
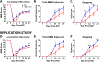
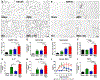
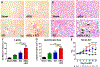
The current high obesity rates mean that neurological injuries are increasingly sustained on a background of systemic pathology, including liver inflammation, which likely has a negative impact on outcomes. Because obesity involves complex pathology, the effect of hepatic inflammation alone on neurological recovery is unknown. Thus, here we used a gain-of-function model to test if liver inflammation worsens outcome from spinal cord injury (SCI) in rats. Results show liver inflammation concomitant with SCI exacerbated intraspinal pathology and impaired locomotor recovery. Hepatic inflammation also potentiated SCI-induced non-alcoholic steatohepatitis (NASH), endotoxemia and insulin resistance. Circulating and cerebrospinal levels of the liver-derived protein Fetuin-A were higher in SCI rats with liver inflammation, and, when microinjected into intact spinal cords, Fetuin-A caused macrophage activation and neuron loss. Thus, liver inflammation functions as a disease modifying factor to impair recovery from SCI, and Fetuin-A is a potential neuropathological mediator. Since SCI alone induces acute liver inflammation, the liver may be a novel clinical target for improving recovery from SCI.
Keywords: ALT; Fatty liver disease; Hepatocyte ballooning; Iron; Kupffer cells; Metabolic syndrome; Obesity; TLR4; TNF; White matter sparing.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Authors disclosure statement
No competing interests exist.
Declaration of Competing Interest
Authors declare there are no competing interests.
Figures









Similar articles
-
Spinal cord injury-induced metabolic impairment and steatohepatitis develops in non-obese rats and is exacerbated by premorbid obesity.Exp Neurol. 2024 Sep;379:114847. doi: 10.1016/j.expneurol.2024.114847. Epub 2024 Jun 8. Exp Neurol. 2024. PMID: 38852834 Free PMC article.
-
Dietary Green Tea Extract Prior to Spinal Cord Injury Prevents Hepatic Iron Overload but Does Not Improve Chronic Hepatic and Spinal Cord Pathology in Rats.J Neurotrauma. 2018 Dec 15;35(24):2872-2882. doi: 10.1089/neu.2018.5771. Epub 2018 Sep 27. J Neurotrauma. 2018. PMID: 30084733 Free PMC article.
-
The immunomodulator decoy receptor 3 improves locomotor functional recovery after spinal cord injury.J Neuroinflammation. 2016 Jun 17;13(1):154. doi: 10.1186/s12974-016-0623-6. J Neuroinflammation. 2016. PMID: 27316538 Free PMC article.
-
Hepatic dysfunction after spinal cord injury: A vicious cycle of central and peripheral pathology?Exp Neurol. 2020 Mar;325:113160. doi: 10.1016/j.expneurol.2019.113160. Epub 2019 Dec 18. Exp Neurol. 2020. PMID: 31863731 Review.
-
The paradox of chronic neuroinflammation, systemic immune suppression, autoimmunity after traumatic chronic spinal cord injury.Exp Neurol. 2014 Aug;258:121-129. doi: 10.1016/j.expneurol.2014.04.023. Exp Neurol. 2014. PMID: 25017893 Free PMC article. Review.
Cited by
-
Muscle-restricted knockout of connexin 43 and connexin 45 accelerates and improves locomotor recovery after contusion spinal cord injury.Front Physiol. 2024 Oct 25;15:1486691. doi: 10.3389/fphys.2024.1486691. eCollection 2024. Front Physiol. 2024. PMID: 39524606 Free PMC article.
-
Updating perspectives on spinal cord function: motor coordination, timing, relational processing, and memory below the brain.Front Syst Neurosci. 2024 Feb 20;18:1184597. doi: 10.3389/fnsys.2024.1184597. eCollection 2024. Front Syst Neurosci. 2024. PMID: 38444825 Free PMC article. Review.
-
System failure: Systemic inflammation following spinal cord injury.Eur J Immunol. 2024 Jan;54(1):e2250274. doi: 10.1002/eji.202250274. Epub 2023 Oct 19. Eur J Immunol. 2024. PMID: 37822141 Free PMC article. Review.
-
Immunoglobulin G Is Increased in the Injured Spinal Cord in a Sex- and Age-Dependent Manner.J Neurotrauma. 2022 Aug;39(15-16):1090-1098. doi: 10.1089/neu.2022.0011. Epub 2022 Jul 13. J Neurotrauma. 2022. PMID: 35373588 Free PMC article.
-
Spinal cord injury-induced metabolic impairment and steatohepatitis develops in non-obese rats and is exacerbated by premorbid obesity.Exp Neurol. 2024 Sep;379:114847. doi: 10.1016/j.expneurol.2024.114847. Epub 2024 Jun 8. Exp Neurol. 2024. PMID: 38852834 Free PMC article.
References
-
- Ballestri S, Zona S, Targher G, Romagnoli D, Baldelli E, Nascimbeni F, Roverato A, Guaraldi G, Lonardo A, 2016. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J. Gastroenterol. Hepatol 31, 936–944. 10.1111/jgh.13264. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

