Isopentenyltransferases as master regulators of crop performance: their function, manipulation, and genetic potential for stress adaptation and yield improvement
- PMID: 33934489
- PMCID: PMC8313133
- DOI: 10.1111/pbi.13603
Isopentenyltransferases as master regulators of crop performance: their function, manipulation, and genetic potential for stress adaptation and yield improvement
Abstract
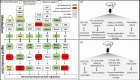
Isopentenyltransferase (IPT) in plants regulates a rate-limiting step of cytokinin (CTK) biosynthesis. IPTs are recognized as key regulators of CTK homeostasis and phytohormone crosstalk in both biotic and abiotic stress responses. Recent research has revealed the regulatory function of IPTs in gene expression and metabolite profiles including source-sink modifications, energy metabolism, nutrient allocation and storage, stress defence and signalling pathways, protein synthesis and transport, and membrane transport. This suggests that IPTs play a crucial role in plant growth and adaptation. In planta studies of IPT-driven modifications indicate that, at a physiological level, IPTs improve stay-green characteristics, delay senescence, reduce stress-induced oxidative damage and protect photosynthetic machinery. Subsequently, these improvements often manifest as enhanced or stabilized crop yields and this is especially apparent under environmental stress. These mechanisms merit consideration of the IPTs as 'master regulators' of core cellular metabolic pathways, thus adjusting plant homeostasis/adaptive responses to altered environmental stresses, to maximize yield potential. If their expression can be adequately controlled, both spatially and temporally, IPTs can be a key driver for seed yield. In this review, we give a comprehensive overview of recent findings on how IPTs influence plant stress physiology and yield, and we highlight areas for future research.
Keywords: IPT; abiotic stress; biotic stress; cytokinin; phytohormone; plant yield; stress response.
© 2021 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
All authors agree to authorship and submission of the manuscript for peer review. The authors report no commercial or proprietary interest in any product or concept discussed in this article.
Figures


References
-
- Alvarez, S. , Marsh, E.L. , Schroeder, S.G. and Schachtman, D.P. (2008) Metabolomic and proteomic changes in the xylem sap of maize under drought. Plant, Cell Environ. 31, 325–340. - PubMed
-
- Argueso, C.T. , Ferreira, F.J. and Kieber, J.J. (2009) Environmental perception avenues: the interaction of cytokinin and environmental response pathways. Plant, Cell Environ. 32, 1147–1160. - PubMed
-
- Atkins, C.A. , Emery, R.N. and Smith, P.M. (2011) Consequences of transforming narrow leafed lupin (Lupinus angustifolius [L.]) with an ipt gene under control of a flower‐specific promoter. Transgenic Res. 20, 1321–1332. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

