Pathogenicity of an African swine fever virus strain isolated in Vietnam and alternative diagnostic specimens for early detection of viral infection
- PMID: 33934707
- PMCID: PMC8091783
- DOI: 10.1186/s40813-021-00215-0
Pathogenicity of an African swine fever virus strain isolated in Vietnam and alternative diagnostic specimens for early detection of viral infection
Abstract
Background: African swine fever (ASF), caused by the ASF virus (ASFV), was first reported in Vietnam in 2019 and spread rapidly thereafter. Better insights into ASFV characteristics and early detection by surveillance could help control its spread. However, the pathogenicity and methods for early detection of ASFV isolates from Vietnam have not been established. Therefore, we investigated the pathogenicity of ASFV and explored alternative sampling methods for early detection.
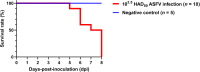
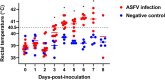
Results: Ten pigs were intramuscularly inoculated with an ASFV strain from Vietnam (titer, 103.5 HAD50/mL), and their temperature, clinical signs, and virus excretion patterns were recorded. In addition, herd and environmental samples were collected daily. The pigs died 5-8 days-post-inoculation (dpi), and the incubation period was 3.7 ± 0.5 dpi. ASFV genome was first detected in the blood (2.2 ± 0.8) and then in rectal (3.1 ± 0.7), nasal (3.2 ± 0.4), and oral (3.6 ± 0.7 dpi) swab samples. ASFV was detected in oral fluid samples collected using a chewed rope from 3 dpi. The liver showed the highest viral loads, and ear tissue also exhibited high viral loads among 11 tissues obtained from dead pigs. Overall, ASFV from Vietnam was classified as peracute to acute form. The rope-based oral fluid collection method could be useful for early ASFV detection and allows successful ASF surveillance in large pig farms. Furthermore, ear tissue samples might be a simple alternative specimen for diagnosing ASF infection in dead pigs.
Conclusions: Our data provide valuable insights into the characteristics of a typical ASFV strain isolated in Vietnam and suggest an alternative, non-invasive specimen collection strategy for early detection.
Keywords: African swine fever; Alternative diagnostic specimen; Clinical signs; Incubation period; Pathogenicity; Vietnam; Virus excretion pattern.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures
References
-
- OIE. Technical disease card of African swine fever; 2019. https://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/p.... Accessed June 2019.
-
- Dixon LK, Alonso C, Escribano J, Martins C, Revilla Y, Salas ML. Family Asfarviridae. In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ, editors. Classification and nomenclature of viruses. Nine Report of the International Committee on Taxonomy of viruses. Amsterdam: Elsevier Academic Press; 2012. pp. 153–62.
-
- Achenbach JE, Gallardo C, Nieto-Pelegrín E, Rivera‐Arroyo B, Degefa‐Negi T, Arias M, et al. Identification of a new genotype of African swine fever virus in domestic pigs from Ethiopia. Transbound Emerg Dis. 2017;64:1393–404. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

