Adequacy of alcohol-based handrub solution production practice in response to COVID-19 in public hospitals found in Addis Ababa, Ethiopia: a multicentered cross-sectional study
- PMID: 33934722
- PMCID: PMC8088824
- DOI: 10.1186/s40545-021-00321-y
Adequacy of alcohol-based handrub solution production practice in response to COVID-19 in public hospitals found in Addis Ababa, Ethiopia: a multicentered cross-sectional study
Abstract
Background: Proper hand hygiene using alcohol-based handrub (ABHR) is an effective preventive approach for the current Coronavirus Disease 2019 (COVID-19) pandemic and other infections. World Health Organization recommends local production of ABHR solution in healthcare settings which provides a feasible alternative to the use of relatively expensive commercially produced hand sanitizers. The aim of this study was to explore the adequacy of ABHR solution production practice in response to COVID-19 in public hospitals of Addis Ababa, Ethiopia.
Methods: A cross-sectional observational study was applied using assessment checklist for evaluation of the adequacy of ABHR production practice in compounding units of public hospitals. The evaluation was done with regard to the standard requirements as per the checklist. Statistical Package for Social Sciences (SPSS) version 23 was used for data entry and analysis. Descriptive statistics was employed for analyses of data and categorical variables were described by frequencies and percentages.
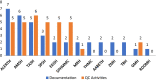
Results: Out of the 13 public hospitals observed in the study, 11 facilities had dedicated premises for compounding of ABHR solution. Seven facilities determined the concentration of ethanol in ABHR solution using alcoholmeters. Only one health facility had a titration kit and performed a strength test for the hydrogen peroxide raw material. Thermal and chemical disinfection processes were practiced for cleaning of recycled dispensing bottles only in 3 and 2 hospitals, respectively. Most of the hospitals (11 facilities) had standard operating procedures (SOPs) for production, but the majority lack SOPs for beyond-use-date assignment (11 facilities), premise and equipment cleaning (12 facilities), and disinfection of recycled bottles (12 facilities).
Conclusion: Most hospitals have fulfilled the majority requirements of premises required for compounding of ABHR solution in their facilities. Five hospitals did not verify the concentration of ethanol in the ABHR solution which might affect the effectiveness of the product. Generally, lower compliance of the majority studied hospitals to good compounding practice was observed during ABHR solution production especially for product preparation, quality control, and documentation.
Keywords: ABHR; COVID-19; Compounding; Documentation; Hospitals; Quality.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Coronavirus Updates, 2020. https://www.worldometers.info/coronavirus/. Accessed 26 Dec 2020.
-
- Ethiopia Confirmed The First Case of COVID-19-FMOH. http://www.moh.gov.et/ejcc/en/node/194. Accessed 24 Jul 2020.
LinkOut - more resources
Full Text Sources
Other Literature Sources
