Accuracy of rapid antigen detection test for nasopharyngeal swab specimens and saliva samples in comparison with RT-PCR and viral culture for SARS-CoV-2 detection
- PMID: 33934920
- PMCID: PMC8052479
- DOI: 10.1016/j.jiac.2021.04.010
Accuracy of rapid antigen detection test for nasopharyngeal swab specimens and saliva samples in comparison with RT-PCR and viral culture for SARS-CoV-2 detection
Abstract
Introduction: Rapid antigen detection (RAD) tests are convenient tools for detecting the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in clinics, and testing using saliva samples could decrease the risk of infection during sample collection. This study aimed to assess the accuracy of the SARS-CoV-2 RAD for testing of nasopharyngeal swab specimens and saliva samples in comparison with the RT-PCR tests and viral culture for detecting viable virus.
Methods: One hundred seventeen nasopharyngeal swab specimens and 73 saliva samples with positive results on RT-PCR were used. Residual samples were assayed using a commercially available RAD test immediately, and its positivity was determined at various time points during the clinical course. The concordance between 54 nasopharyngeal swab samples and saliva samples that were collected simultaneously was determined. Viral culture was performed on 117 samples and compared with the results of the RAD test.
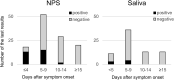
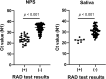
Results: The positive rate of RAD test using saliva samples was low throughout the clinical course. Poor concordance was observed between nasopharyngeal swab specimens and saliva samples (75.9%, kappa coefficient 0.310). However, a substantially high concordance between the RAD test and viral culture was observed in both nasopharyngeal swab specimens (86.8%, kappa coefficient 0.680) and saliva samples (95.1%, kappa coefficient 0.643).
Conclusions: The sensitivity of the SARS-CoV-2 RAD test was insufficient, particularly for saliva samples. However, a substantially high concordance with viral culture suggests its potential utility as an auxiliary test for estimating SARS-CoV-2 viability.
Keywords: Nasopharyngeal swabs; Rapid antigen detection test; SARS-CoV-2; Saliva; Viral culture.
Copyright © 2021 Japanese Society of Chemotherapy and The Japanese Association for Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
None.
Figures
References
-
- Nagura-Ikeda M., Imai K., Tabata S., Miyoshi K., Murahara N., Mizuno T., et al. Clinical evaluation of self-collected saliva by quantitative reverse transcription-pcr (rt-qpcr), direct rt-qpcr, reverse transcription-loop-mediated isothermal amplification, and a rapid antigen test to diagnose covid-19. J Clin Microbiol. 2020;58 - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

