In vitro susceptibility of human Blastocystis subtypes to simeprevir
- PMID: 33935570
- PMCID: PMC8071969
- DOI: 10.1016/j.sjbs.2021.01.050
In vitro susceptibility of human Blastocystis subtypes to simeprevir
Abstract
Introduction and aim: Blastocystis is a common enteric parasite, having a worldwide distribution. Many antimicrobial agents are effective against it, yet side effects and drug resistance have been reported. Thus, ongoing trials are being conducted for exploring anti-Blastocystis alternatives. Proteases are attractive anti-protozoal drug targets, having documented roles in Blastocystis. Serine proteases are present in both hepatitis C virus and Blastocystis. Since drug repositioning is quite trendy, the in vitro efficacy of simeprevir (SMV), an anti-hepatitis serine protease inhibitor, against Blastocystis was investigated in the current study.
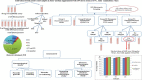
Methods: Stool samples were collected from patients, Alexandria, Egypt. Concentrated stools were screened using direct smears, trichrome, and modified Ziehl-Neelsen stains to exclude parasitic co-infections. Positive stool isolates were cultivated, molecularly subtyped for assessing the efficacy of three SMV doses (100,150, and 200 μg/ml) along 72 hours (h), on the most common subtype, through monitoring parasite growth, viability, re-culture, and also via ultrastructure verification. The most efficient dose and duration were later tested on other subtypes.
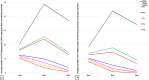
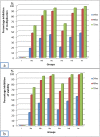
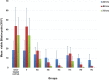
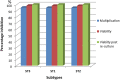
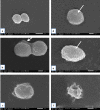
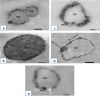
Results: Results revealed that Blastocystis was detected in 54.17% of examined samples. Molecularly, ST3 predominated (62%), followed by ST1 (8.6%) and ST2 (3.4%). Ascending concentrations of SMV progressively inhibited growth, viability, and re-culture of treated Blastocystis, with a non-statistically significant difference when compared to the therapeutic control metronidazole (MTZ). The most efficient dose and duration against ST3 was 150 µg/ml for 72 h. This dose inhibited the growth of ST3, ST1, and ST2 with percentages of 95.19%, 94.83%, and 94.74%, successively and viability with percentages of 98.30%, 98.09%, and 97.96%, successively. This dose abolished Blastocystis upon re-culturing. Ultra-structurally, SMV induced rupture of Blastocystis cell membrane leading to necrotic death, versus the reported apoptotic death caused by MTZ. In conclusion, 150 µg/ml SMV for 72 h proved its efficacy against ST1, ST2, and ST3 Blastocystis, thus sparing the need for pre-treatment molecular subtyping in developing countries.
Keywords: Blastocystis subtypes; CV, central vacuole; DMSO, Dimethyl Sulfoxide; IBS, irritable bowel syndrome; In vitro; MLO, Mitochondrion-like organelle; MTZ, Metronidazole; PCR, Polymerase chain reaction; Re-culture; SEM, Scanning electron microscopy; SMV, Simeprevir; ST, subtypes; Simeprevir; TEM, Transmission electron microscopy; Ultrastructure; Viability.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Abaza S., Rayan H., Soliman R., Nemr N., Mokhtar A. Subtype analysis of Blastocystis spp. isolates from symptomatic and asymptomatic patients in Suez Canal University Hospitals, Ismailia, Egypt. Parasitol. United. J. 2014;7(1):56. doi: 10.4103/1687-7942.139691. - DOI
-
- Abou El Naga I.F., Negm A.Y. Morphology, histochemistry and infectivity of Blastocystis hominis cyst. J. Egypt. Soc. Parasitol. 2001;31(2):627–635. PMID: 11478461. - PubMed
-
- Adao D.E., Rivera W.L. Recent advances in Blastocystis sp. research. Philipp. Sci. Lett. 2018;11(1):39–60.
-
- Al-Mohammed H.I., Hussein E.M., Aboulmagd E. Effect of Green Tea Extract and Cysteine Proteases Inhibitor (E-64) on Symptomatic Genotypes of Blastocystis hominis in vitro and in Infected Animal Model. Int. J. Curr. Microbiol. App. Sci. 2013;2(12):228–239.
LinkOut - more resources
Full Text Sources
Other Literature Sources

