Pharmacokinetics, Bioavailability, Excretion and Metabolism Studies of Akebia Saponin D in Rats: Causes of the Ultra-Low Oral Bioavailability and Metabolic Pathway
- PMID: 33935711
- PMCID: PMC8082176
- DOI: 10.3389/fphar.2021.621003
Pharmacokinetics, Bioavailability, Excretion and Metabolism Studies of Akebia Saponin D in Rats: Causes of the Ultra-Low Oral Bioavailability and Metabolic Pathway
Abstract
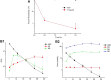
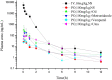
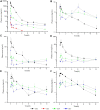
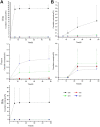
Background: Akebia saponin D (ASD) has a variety of biological activities and great medicinal potential, but its oral bioavailability is so low as to limit its development. Its pharmacokinetic profiles and excretion and metabolism in vivo have not been fully elucidated. This study was an attempt in this area. Methods: A simple LC-MS/MS method to simultaneously quantify ASD and its metabolites M1∼M5 in rat plasma, feces, urine and bile was established with a negative ESI model using dexketoprofen as the internal standard. Meanwhile, the UPLC-HR/MS system was used to screen all possible metabolites in the urine, feces and bile of rats, as compared with blank samples collected before administration. Absolute quantitative analysis was for M0, M3, M4, and M5, while semi-quantitative analysis was for M1, M2, and Orbitrap data. Results: The AUC0-t values after intravenous administration of 10 mg/kg and intragastrical administration of 100 mg/kg ASD were 19.05 ± 8.64 and 0.047 ± 0.030 h*μg/ml respectively. The oral bioavailability was determined to be extremely low (0.025%) in rats. The exposure of M4 and M5 in the oral group was higher than that of M0 in the terminal phase of the plasma concentration time profile, and ASD was stable in the liver microsome incubation system of rats, but metabolism was relatively rapid during anaerobic incubation of intestinal contents of rats, suggesting that the low bioavailability of ASD might have been attributed to the poor gastrointestinal permeability and extensive pre-absorption degradation rather than to the potent first pass metabolism. This assertion was further verified by a series of intervention studies, where improvement of lipid solubility and intestinal permeability as well as inhibition of intestinal flora increased the relative bioavailability to different extents without being changed by P-gp inhibition. After intravenous administration, the cumulative excretion rates of ASD in the urine and bile were 14.79 ± 1.87%, and 21.76 ± 17.61% respectively, but only 0.011% in feces, suggesting that the urine and bile were the main excretion pathways and that there was a large amount of biotransformation in the gastrointestinal tract. Fifteen possible metabolites were observed in the urine, feces and bile. The main metabolites were ASD deglycosylation, demethylation, dehydroxylation, decarbonylation, decarboxylation, hydroxylation, hydroxymethylation, hydroxyethylation and hydrolysis. Conclusion: The pharmacokinetics, bioavailability, metabolism and excretion of ASD in rats were systematically evaluated for the first time in this study. It has been confirmed that the ultra-low oral bioavailability is due to poor gastrointestinal permeability, extensive pre-absorption degradation and biotransformation. ASD after iv administration is not only excreted by the urine and bile, but possibly undergoes complex metabolic elimination.
Keywords: Akebia saponin D; LC–MS/MS; bioavailability; excretion; metabolism; pharmacokinetics.
Copyright © 2021 Li, Peng, Li, Gong, Lv, Liu, Zhang, Yang, Liu, Li and Liu.
Conflict of interest statement
Authors JP, TZ, and JL were employed by the company Guollence Pharmaceutical Technology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
In vivo and in vitro metabolism and pharmacokinetics of cholinesterase inhibitor deoxyvasicine from aerial parts of Peganum harmala Linn in rats via UPLC-ESI-QTOF-MS and UPLC-ESI-MS/MS.J Ethnopharmacol. 2019 May 23;236:288-301. doi: 10.1016/j.jep.2019.03.020. Epub 2019 Mar 11. J Ethnopharmacol. 2019. PMID: 30872168
-
Gender-related pharmacokinetics and absolute bioavailability of diosbulbin B in rats determined by ultra-performance liquid chromatography-tandem mass spectrometry.J Ethnopharmacol. 2013 Oct 7;149(3):810-5. doi: 10.1016/j.jep.2013.08.010. Epub 2013 Aug 14. J Ethnopharmacol. 2013. PMID: 23954278
-
A novel double-tracer technique to characterize absorption, distribution, metabolism and excretion (ADME) of [14C]tofogliflozin after oral administration and concomitant intravenous microdose administration of [13C]tofogliflozin in humans.Clin Pharmacokinet. 2013 Jun;52(6):463-73. doi: 10.1007/s40262-013-0051-z. Clin Pharmacokinet. 2013. PMID: 23494983 Clinical Trial.
-
Pharmacokinetics and pharmacodynamics in clinical use of scopolamine.Ther Drug Monit. 2005 Oct;27(5):655-65. doi: 10.1097/01.ftd.0000168293.48226.57. Ther Drug Monit. 2005. PMID: 16175141 Review.
-
Structural characterization of the metabolites of orally ingested hederasaponin B, a natural saponin that is isolated from Acanthopanax senticosus leaves by liquid chromatography-mass spectrometry.J Pharm Biomed Anal. 2021 Apr 15;197:113929. doi: 10.1016/j.jpba.2021.113929. Epub 2021 Jan 29. J Pharm Biomed Anal. 2021. PMID: 33618133 Review.
Cited by
-
Simultaneous Component Analysis of Akebia quinata Seeds (Lardizabalaceae) by Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry for Quality and Cytotoxicity Assessment.Plants (Basel). 2025 Feb 21;14(5):669. doi: 10.3390/plants14050669. Plants (Basel). 2025. PMID: 40094529 Free PMC article.
-
Advancements and challenges in pharmacokinetic and pharmacodynamic research on the traditional Chinese medicine saponins: a comprehensive review.Front Pharmacol. 2024 May 7;15:1393409. doi: 10.3389/fphar.2024.1393409. eCollection 2024. Front Pharmacol. 2024. PMID: 38774213 Free PMC article. Review.
-
Simultaneous Determination and Pharmacokinetics Study of Three Triterpenes from Sanguisorba officinalis L. in Rats by UHPLC-MS/MS.Molecules. 2022 Aug 24;27(17):5412. doi: 10.3390/molecules27175412. Molecules. 2022. PMID: 36080179 Free PMC article.
-
The Importance of Murine Models in Determining In Vivo Pharmacokinetics, Safety, and Efficacy in Antimalarial Drug Discovery.Pharmaceuticals (Basel). 2025 Mar 18;18(3):424. doi: 10.3390/ph18030424. Pharmaceuticals (Basel). 2025. PMID: 40143200 Free PMC article. Review.
-
Harnessing Thalassochemicals: Marine Saponins as Bioactive Agents in Nutraceuticals and Food Technologies.Mar Drugs. 2025 May 26;23(6):227. doi: 10.3390/md23060227. Mar Drugs. 2025. PMID: 40559636 Free PMC article. Review.
References
-
- Amer S. M., Kadi A. A., Darwish H. W., Attwa M. W. (2017). Identification and characterization of in vitro phase I and reactive metabolites of masitinib using a LC-MS/MS method: bioactivation pathway elucidation. J. RSC Adv. 7, 4479–4491. 10.1039/C6RA25767D - DOI
-
- Chinese Pharmacopoeia Commission (2005). S.Pharmacopoeia of the people's republic of hina. Beijing: Chemical Industry Press, 231.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

