Efficacy of Antiangiogenic Drugs in the Treatment of Diabetic Macular Edema: A Bayesian Network Analysis
- PMID: 33935727
- PMCID: PMC8082725
- DOI: 10.3389/fphar.2021.637667
Efficacy of Antiangiogenic Drugs in the Treatment of Diabetic Macular Edema: A Bayesian Network Analysis
Abstract
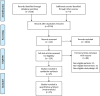
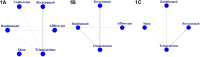
Aims: To compare the efficacy of five kinds of antiangiogenic drugs in the treatment of diabetic macular edema Methods: A comprehensive search of seven databases without language restrictions includes PubMed, EMBASE, Web of Science, CBM, the Cochrane Library, CNKI, and WanFang date. All literature used was published before October 2020. Eligible randomized trials were screened for inclusion in this study, and Bayesian framework was used to perform a network meta-analysis (NMA). Data on the mean change of best-corrected visual acuity (BCVA), central macular thickness (CMT) and intraocular pressure (IOP) at 6 months were extracted. Results: 25 randomized controlled trials (RCTs) that covered 2214 eyes, which received treatment of more than 3 months durations were included. In the pooled pair-wise meta-analysis, there was no statistically significant difference between all treatments. The same result was observed in the network meta-analysis with 0-37.82% Global I-squared. For BCVA at 6 months, conbercept and ranibizumab may be favorable than bevacizumab, aflibercept, triamcinolone acetonide and sham injections according to the ranking probabilities. As for CMT at 6 months, ranibizumab may be the most effective compared to bevacizumab, aflibercept and triamcinolone acetonide. In terms of IOP at 6 months, ranibizumab have better effect than bevacizumab, triamcinolone acetonide and sham injections. The results of sensitivity analysis also confirm it. Conclusion: The analysis confirms that ranibizumab may be the most favorable for BCVA improvement and have a stronger efficacy in decreasing CMT and IOP than other drugs when taking all the indicators into consideration. This conclusion may provide clinical evidence to guide treatment decisions. However, more high-quality randomized controlled trials will be necessary to further confirm this.
Keywords: antiangiogenic drugs; best-corrected visual acuity; central macular thickness; diabetic macular edema; intraocular pressure; network meta-analysis.
Copyright © 2021 Zhang, Liu, Wang, Li, Zhang, Zhang and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

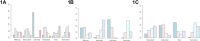
References
-
- Baghi A., Jabbarpoor Bonyadi M. H., Ramezani A., Azarmina M., Moradian S., Dehghan M. H., et al. (2017). Two doses of intravitreal ziv-aflibercept versus bevacizumab in treatment of diabetic macular edema: a three-armed, double-blind randomized trial. Ophthalmol. Retina 1 (2), 103–110. 10.1016/j.oret.2016.08.007 - DOI - PubMed
-
- Ekinci M., Ceylan E., Çakıcı Ö., Tanyıldız B., Olcaysu O., Çağatay H. H. (2014). Treatment of macular edema in diabetic retinopathy: comparison of the efficacy of intravitreal bevacizumab and ranibizumab injections. Expert Rev. Ophthalmol. 9 (2), 139–143. 10.1586/17469899.2014.900439 - DOI
-
- Genentech, Inc. (2007). A study of ranibizumab injection in subjects with clinically significant macular edema (ME) with center involvement secondary to diabetes mellitus (RISE). Available at: https://clinicaltrialsgov/show/NCT00473330 2007 (Accessed May 15, 2007).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

