Therapies for Chronic Allograft Rejection
- PMID: 33935762
- PMCID: PMC8082459
- DOI: 10.3389/fphar.2021.651222
Therapies for Chronic Allograft Rejection
Abstract
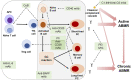
Remarkable advances have been made in the pathophysiology, diagnosis, and treatment of antibody-mediated rejection (ABMR) over the past decades, leading to improved graft outcomes. However, long-term failure is still high and effective treatment for chronic ABMR, an important cause of graft failure, has not yet been identified. Chronic ABMR has a relatively different phenotype from active ABMR and is a slowly progressive disease in which graft injury is mainly caused by de novo donor specific antibodies (DSA). Since most trials of current immunosuppressive therapies for rejection have focused on active ABMR, treatment strategies based on those data might be less effective in chronic ABMR. A better understanding of chronic ABMR may serve as a bridge in establishing treatment strategies to improve graft outcomes. In this in-depth review, we focus on the pathophysiology and characteristics of chronic ABMR along with the newly revised Banff criteria in 2017. In addition, in terms of chronic ABMR, we identify the reasons for the resistance of current immunosuppressive therapies and look at ongoing research that could play a role in setting better treatment strategies in the future. Finally, we review non-invasive biomarkers as tools to monitor for rejection.
Keywords: antibody formation; antirejection therapy; graft rejection; kidney transplantation; transplantation immunology.
Copyright © 2021 Kim and Brennan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

