Luteolin Ameliorates Experimental Pulmonary Arterial Hypertension via Suppressing Hippo-YAP/PI3K/AKT Signaling Pathway
- PMID: 33935785
- PMCID: PMC8082250
- DOI: 10.3389/fphar.2021.663551
Luteolin Ameliorates Experimental Pulmonary Arterial Hypertension via Suppressing Hippo-YAP/PI3K/AKT Signaling Pathway
Abstract
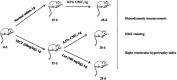
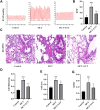
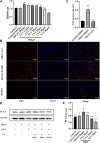
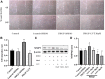
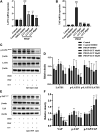
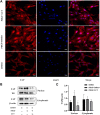
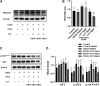
Luteolin is a flavonoid compound with a variety of pharmacological effects. In this study, we explored the effects of luteolin on monocrotaline (MCT) induced rat pulmonary arterial hypertension (PAH) and underlying mechanisms. A rat PAH model was generated through MCT injection. In this model, luteolin improved pulmonary vascular remodeling and right ventricular hypertrophy, meanwhile, luteolin could inhibit the proliferation and migration of pulmonary artery smooth muscle cells induced by platelet-derived growth factor-BB (PDGF-BB) in a dose-dependent manner. Moreover, our results showed that luteolin could downregulate the expression of LATS1 and YAP, decrease YAP nuclear localization, reduce the expression of PI3K, and thereby restrain the phosphorylation of AKT induced by PDGF-BB. In conclusion, luteolin ameliorated experimental PAH, which was at least partly mediated through suppressing HIPPO-YAP/PI3K/AKT signaling pathway. Therefore, luteolin might become a promising candidate for treatment of PAH.
Keywords: PI3K; Pulmonary arterial hypertension; akt; hippo-yap; luteolin.
Copyright © 2021 Zuo, Liu, Zeng, Xiao, Wu, Yang, Li, Song, Xiao and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Anson D. M., Wilcox R. M., Huseman E. D., Stump T. A., Paris R. L., Darkwah B. O., et al. (2018). Luteolin decreases epidermal growth factor receptor-mediated cell proliferation and induces apoptosis in glioblastoma cell lines. Basic Clin. Pharmacol. Toxicol. 123, 678–686. 10.1111/bcpt.13077 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

