Lipoarabinomannan as a Point-of-Care Assay for Diagnosis of Tuberculosis: How Far Are We to Use It?
- PMID: 33935997
- PMCID: PMC8081860
- DOI: 10.3389/fmicb.2021.638047
Lipoarabinomannan as a Point-of-Care Assay for Diagnosis of Tuberculosis: How Far Are We to Use It?
Abstract
Tuberculosis (TB) is still a severe public health problem; the current diagnostic tests have limitations that delay treatment onset. Lipoarabinomannan (LAM) is a glycolipid that is a component of the cell wall of the bacillus Mycobacterium tuberculosis, the etiologic agent of TB. This glycolipid is excreted as a soluble form in urine. The World Health Organization has established that the design of new TB diagnostic methods is one of the priorities within the EndTB Strategy. LAM has been suggested as a biomarker to develop diagnostic tests based on its identification in urine, and it is one of the most prominent candidates to develop point-of-care diagnostic test because urine samples can be easily collected. Moreover, LAM can regulate the immune response in the host and can be found in the serum of TB patients, where it probably affects a wide variety of host cell populations, consequently influencing the quality of both innate and adaptive immune responses during TB infection. Here, we revised the evidence that supports that LAM could be used as a tool for the development of new point-of-care tests for TB diagnosis, and we discussed the mechanisms that could contribute to the low sensitivity of diagnostic testing.
Keywords: immune response; immunoregulation; lipoarabinomannan; point-of-care assay; tuberculosis.
Copyright © 2021 Flores, Cancino and Chavez-Galan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
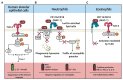
Figures



References
-
- Alonso H., Parra J., Malaga W., Payros D., Liu C.-F., Berrone C., et al. (2017). Protein O-mannosylation deficiency increases LprG-associated lipoarabinomannan release by Mycobacterium tuberculosis and enhances the TLR2-associated inflammatory response. Sci. Rep. 7:7913. 10.1038/s41598-017-08489-7, PMID: - DOI - PMC - PubMed
-
- Amin A. G., De P., Spencer J. S., Brennan P. J., Daum J., Andre B. G., et al. (2018). Detection of lipoarabinomannan in urine and serum of HIV-positive and HIV-negative TB suspects using an improved capture-enzyme linked immuno absorbent assay and gas chromatography/mass spectrometry. Tuberculosis 111, 178–187. 10.1016/j.tube.2018.06.004, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

