Reductive Cytochrome P450 Reactions and Their Potential Role in Bioremediation
- PMID: 33936006
- PMCID: PMC8081977
- DOI: 10.3389/fmicb.2021.649273
Reductive Cytochrome P450 Reactions and Their Potential Role in Bioremediation
Abstract
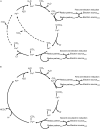
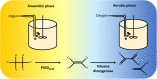
Cytochrome P450 enzymes, or P450s, are haem monooxygenases renowned for their ability to insert one atom from molecular oxygen into an exceptionally broad range of substrates while reducing the other atom to water. However, some substrates including many organohalide and nitro compounds present little or no opportunity for oxidation. Under hypoxic conditions P450s can perform reductive reactions, contributing electrons to drive reductive elimination reactions. P450s can catalyse dehalogenation and denitration of a range of environmentally persistent pollutants including halogenated hydrocarbons and nitroamine explosives. P450-mediated reductive dehalogenations were first discovered in the context of human pharmacology but have since been observed in a variety of organisms. Additionally, P450-mediated reductive denitration of synthetic explosives has been discovered in bacteria that inhabit contaminated soils. This review will examine the distribution of P450-mediated reductive dehalogenations and denitrations in nature and discuss synthetic biology approaches to developing P450-based reagents for bioremediation.
Keywords: bioremediation; cytochrome P450; dehalogenation; denitration; synthetic biology.
Copyright © 2021 Behrendorff.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Organohalide Respiring Bacteria and Reductive Dehalogenases: Key Tools in Organohalide Bioremediation.Front Microbiol. 2016 Mar 1;7:249. doi: 10.3389/fmicb.2016.00249. eCollection 2016. Front Microbiol. 2016. PMID: 26973626 Free PMC article. Review.
-
Integration of organohalide-respiring bacteria and nanoscale zero-valent iron (Bio-nZVI-RD): A perfect marriage for the remediation of organohalide pollutants?Biotechnol Adv. 2016 Dec;34(8):1384-1395. doi: 10.1016/j.biotechadv.2016.10.004. Epub 2016 Oct 17. Biotechnol Adv. 2016. PMID: 27765723 Review.
-
Metabolism of halogenated alkanes by cytochrome P450 enzymes. Aerobic oxidation versus anaerobic reduction.Chem Asian J. 2014 Apr;9(4):1175-82. doi: 10.1002/asia.201301608. Epub 2014 Feb 5. Chem Asian J. 2014. PMID: 24501011
-
Evolution of Cytochrome P450 Enzymes and Their Redox Partners in Archaea.Int J Mol Sci. 2023 Feb 19;24(4):4161. doi: 10.3390/ijms24044161. Int J Mol Sci. 2023. PMID: 36835573 Free PMC article.
-
Electron transport chains in organohalide-respiring bacteria and bioremediation implications.Biotechnol Adv. 2018 Jul-Aug;36(4):1194-1206. doi: 10.1016/j.biotechadv.2018.03.018. Epub 2018 Apr 6. Biotechnol Adv. 2018. PMID: 29631017 Review.
Cited by
-
Pharmaceutical removal from wastewater by introducing cytochrome P450s into microalgae.Microb Biotechnol. 2024 Jun;17(6):e14515. doi: 10.1111/1751-7915.14515. Microb Biotechnol. 2024. PMID: 38925623 Free PMC article. Review.
-
Toward the development of a molecular toolkit for the microbial remediation of per-and polyfluoroalkyl substances.Appl Environ Microbiol. 2024 Apr 17;90(4):e0015724. doi: 10.1128/aem.00157-24. Epub 2024 Mar 13. Appl Environ Microbiol. 2024. PMID: 38477530 Free PMC article. Review.
-
Toxic Effects of p-Chloroaniline on Cells of Fungus Isaria fumosorosea SP535 and the Role of Cytochrome P450.Toxics. 2025 Jun 16;13(6):506. doi: 10.3390/toxics13060506. Toxics. 2025. PMID: 40559979 Free PMC article.
-
Burning question: Rethinking organohalide degradation strategy for bioremediation applications.Microb Biotechnol. 2024 Aug;17(8):e14539. doi: 10.1111/1751-7915.14539. Microb Biotechnol. 2024. PMID: 39075849 Free PMC article. Review.
-
Analysis of pCl107 a large plasmid carried by an ST25 Acinetobacter baumannii strain reveals a complex evolutionary history and links to multiple antibiotic resistance and metabolic pathways.FEMS Microbes. 2022 Nov 18;3:xtac027. doi: 10.1093/femsmc/xtac027. eCollection 2022. FEMS Microbes. 2022. PMID: 37332503 Free PMC article.
References
-
- Amonette J. E., Jeffers P. M., Qafoku O., Russell C. K., Humphrys D. R., Wietsma T. W., et al. (2012). Abiotic Degradation Rates for Carbon Tetrachloride and Chloroform: Final Report. Available online at: https://www.pnnl.gov/main/publications/external/technical_reports/PNNL-2... (accessed December 17, 2020).
-
- Bandopadhyay R., Haque I., Singh D., Mukhopadhyay K. (2010). Levels and stability of expression of transgenes. Transgenic Crop. Plants 1 145–186. 10.1007/978-3-642-04809-8_5 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

