GABA Production by Human Intestinal Bacteroides spp.: Prevalence, Regulation, and Role in Acid Stress Tolerance
- PMID: 33936013
- PMCID: PMC8082179
- DOI: 10.3389/fmicb.2021.656895
GABA Production by Human Intestinal Bacteroides spp.: Prevalence, Regulation, and Role in Acid Stress Tolerance
Abstract
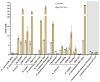
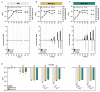
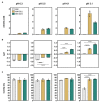
The high neuroactive potential of metabolites produced by gut microbes has gained traction over the last few years, with metagenomic-based studies suggesting an important role of microbiota-derived γ-aminobutyric acid (GABA) in modulating mental health. Emerging evidence has revealed the presence of the glutamate decarboxylase (GAD)-encoding gene, a key enzyme to produce GABA, in the prominent human intestinal genus Bacteroides. Here, we investigated GABA production by Bacteroides in culture and metabolic assays combined with comparative genomics and phylogenetics. A total of 961 Bacteroides genomes were analyzed in silico and 17 metabolically and genetically diverse human intestinal isolates representing 11 species were screened in vitro. Using the model organism Bacteroides thetaiotaomicron DSM 2079, we determined GABA production kinetics, its impact on milieu pH, and we assessed its role in mitigating acid-induced cellular damage. We showed that the GAD-system consists of at least four highly conserved genes encoding a GAD, a glutaminase, a glutamate/GABA antiporter, and a potassium channel. We demonstrated a high prevalence of the GAD-system among Bacteroides with 90% of all Bacteroides genomes (96% in human gut isolates only) harboring all genes of the GAD-system and 16 intestinal Bacteroides strains producing GABA in vitro (ranging from 0.09 to 60.84 mM). We identified glutamate and glutamine as precursors of GABA production, showed that the production is regulated by pH, and that the GAD-system acts as a protective mechanism against acid stress in Bacteroides, mitigating cell death and preserving metabolic activity. Our data also indicate that the GAD-system might represent the only amino acid-dependent acid tolerance system in Bacteroides. Altogether, our results suggest an important contribution of Bacteroides in the regulation of the GABAergic system in the human gut.
Keywords: Bacteroides; GABA; acid stress tolerance; glutamate decarboxylase; gut microbiota.
Copyright © 2021 Otaru, Ye, Mujezinovic, Berchtold, Constancias, Cornejo, Krzystek, de Wouters, Braegger, Lacroix and Pugin.
Conflict of interest statement
Authors LB and TW were employed by the company PharmaBiome AG. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

