Impact of Treatment Regimens on Antibody Response to the SARS-CoV-2 Coronavirus
- PMID: 33936026
- PMCID: PMC8082543
- DOI: 10.3389/fimmu.2021.580147
Impact of Treatment Regimens on Antibody Response to the SARS-CoV-2 Coronavirus
Abstract
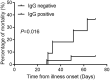
The coronavirus disease 2019 (COVID-19) is widely spread and remains a global pandemic. Limited evidence on the systematic evaluation of the impact of treatment regimens on antibody responses exists. Our study aimed to analyze the role of antibody response on prognosis and determine factors influencing the IgG antibodies' seroconversion. A total of 1,111 patients with mild to moderate COVID-19 symptoms admitted to Leishenshan Hospital in Wuhan were retrospectively analyzed. A serologic SARS-CoV-2 IgM/IgG antibody test was performed on all the patients 21 days after the onset of symptoms. Patient clinical characteristics were compared. In the study, 42 patients progressed to critical illness, with 6 mortalities reported while 1,069 patients reported mild to moderate disease. Advanced age (P = 0.028), gasping (P < 0.001), dyspnea (P = 0.024), and IgG negativity (P = 0.006) were associated with progression to critical illness. The mortality rate in critically ill patients with IgG antibody was 6.45% (95% CI 1.12-22.84%) and 36.36% (95% CI 12.36-68.38%) in patients with no IgG antibody (P = 0.003). Symptomatic patients were more likely to develop IgG antibody responses than asymptomatic patients. Using univariable analysis, fever (P < 0.001), gasping (P = 0.048), cancer (P < 0.001), cephalosporin (P = 0.015), and chloroquine/hydroxychloroquine (P = 0.021) were associated with IgG response. In the multivariable analysis, fever, cancer, cephalosporins, and chloroquine/hydroxychloroquine correlated independently with IgG response. We determined that the absence of SARS-CoV-2 antibody IgG in the convalescent stage had a specific predictive role in critical illness progression. Importantly, risk factors affecting seropositivity were identified, and the effect of antimalarial drugs on antibody response was determined.
Keywords: COVID-19; IgG; SARS-CoV-2; cancer; chloroquine/hydroxychloroquine.
Copyright © 2021 Shang, Liu, Li, Kaweme, Wang and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Qaseem A, Yost J, Etxeandia-Ikobaltzeta I, Humphrey LL. Update Alert 2: Should Clinicians Use Chloroquine or Hydroxychloroquine Alone or in Combination With Azithromycin for the Prophylaxis or Treatment of COVID-19? Living Practice Points From the American College of Physicians. Ann Intern Med (2020) 173:W88–9. 10.7326/L20-1007 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

