Antibody Responses in COVID-19: A Review
- PMID: 33936045
- PMCID: PMC8081880
- DOI: 10.3389/fimmu.2021.633184
Antibody Responses in COVID-19: A Review
Abstract
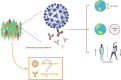
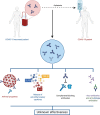
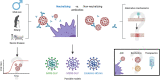
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to spread worldwide as a severe pandemic. Although its seroprevalence is highly variable among territories, it has been reported at around 10%, but higher in health workers. Evidence regarding cross-neutralizing response between SARS-CoV and SARS-CoV-2 is still controversial. However, other previous coronaviruses may interfere with SARS-CoV-2 infection, since they are phylogenetically related and share the same target receptor. Further, the seroconversion of IgM and IgG occurs at around 12 days post onset of symptoms and most patients have neutralizing titers on days 14-20, with great titer variability. Neutralizing antibodies correlate positively with age, male sex, and severity of the disease. Moreover, the use of convalescent plasma has shown controversial results in terms of safety and efficacy, and due to the variable immune response among individuals, measuring antibody titers before transfusion is mostly required. Similarly, cellular immunity seems to be crucial in the resolution of the infection, as SARS-CoV-2-specific CD4+ and CD8+ T cells circulate to some extent in recovered patients. Of note, the duration of the antibody response has not been well established yet.
Keywords: COVID-19; SARS-CoV-2; antibodies; kinetics; neutralization; seroprevalence; therapeutics.
Copyright © 2021 Chvatal-Medina, Mendez-Cortina, Patiño, Velilla and Rugeles.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- World Health Organization . Estimating mortality from COVID-19. In: Scientific brief, 4 August 2020 (2020). p. 5–8. Available at: https://www.who.int/news-room/commentaries/detail/estimating-mortality-f....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

