Novel Biomarkers of Dynamic Blood PD-L1 Expression for Immune Checkpoint Inhibitors in Advanced Non-Small-Cell Lung Cancer Patients
- PMID: 33936103
- PMCID: PMC8085403
- DOI: 10.3389/fimmu.2021.665133
Novel Biomarkers of Dynamic Blood PD-L1 Expression for Immune Checkpoint Inhibitors in Advanced Non-Small-Cell Lung Cancer Patients
Abstract
Background: Immune checkpoint inhibitors (ICIs) have become a high-profile regimen for malignancy recently. However, only a small subpopulation obtains long-term clinical benefit. How to select optimal patients by reasonable biomarkers remains a hot topic.
Methods: Paired tissue samples and blood samples from 51 patients with advanced malignancies were collected for correlation analysis. Dynamic changes in blood PD-L1 (bPD-L1) expression, including PD-L1 mRNA, exosomal PD-L1 (exoPD-L1) protein and soluble PD-L1 (sPD-L1), were detected after 2 months of ICIs treatment in advanced non-small-cell lung cancer (NSCLC) patients. The best cutoff values for progression-free survival (PFS) and overall survival (OS) of all three biomarkers were calculated with R software.
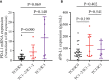
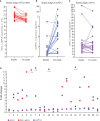
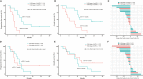
Results: In 51 cases of various malignancies, those with positive tissue PD-L1 (tPD-L1) had significantly higher PD-L1 mRNA than those with negative tPD-L1. In 40 advanced NSCLC patients, those with a fold change of PD-L1 mRNA ≥ 2.04 had better PFS, OS and best objective response (bOR) rate. In addition, a fold change of exoPD-L1 ≥ 1.86 was also found to be associated with better efficacy and OS in a cohort of 21 advanced NSCLC cases. The dynamic change of sPD-L1 was not associated with efficacy and OS. Furthermore, the combination of PD-L1 mRNA and exoPD-L1 could screen better patients for potential benefit from ICIs treatment.
Conclusion: There was a positive correlation between bPD-L1 and tPD-L1 expression. Increased expression of PD-L1 mRNA, exoPD-L1, or both in early stage of ICIs treatment could serve as positive biomarkers of efficacy and OS in advanced NSCLC patients.
Keywords: NSCLC; biomarker; blood PD-L1; exosome; immune checkpoint inhibitors.
Copyright © 2021 Yang, Chen, Gu, Niu, Zhao, Zheng, Xu, Yu, Li, Meng, Chen, Zhuo, Zhang and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Prognostic significance of programmed cell death ligand 1 blood markers in non-small cell lung cancer treated with immune checkpoint inhibitors: a systematic review and meta-analysis.Front Immunol. 2024 Jun 10;15:1400262. doi: 10.3389/fimmu.2024.1400262. eCollection 2024. Front Immunol. 2024. PMID: 38915398 Free PMC article.
-
Soluble PD-L1 as a predictive biomarker in lung cancer: a systematic review and meta-analysis.Future Oncol. 2022 Jan;18(2):261-273. doi: 10.2217/fon-2021-0641. Epub 2021 Dec 7. Future Oncol. 2022. PMID: 34874185
-
Dynamics of Serum Tumor Markers Can Serve as a Prognostic Biomarker for Chinese Advanced Non-small Cell Lung Cancer Patients Treated With Immune Checkpoint Inhibitors.Front Immunol. 2020 Jun 10;11:1173. doi: 10.3389/fimmu.2020.01173. eCollection 2020. Front Immunol. 2020. PMID: 32587591 Free PMC article.
-
The effects of antibiotics on the efficacy of immune checkpoint inhibitors in patients with non-small-cell lung cancer differ based on PD-L1 expression.Eur J Cancer. 2021 May;149:73-81. doi: 10.1016/j.ejca.2021.02.040. Epub 2021 Apr 7. Eur J Cancer. 2021. PMID: 33838391
-
Delta-He as a Novel Predictive and Prognostic Biomarker in Patients With NSCLC Treated With PD-1/PD-L1 Inhibitors.Cancer Med. 2025 Apr;14(7):e70826. doi: 10.1002/cam4.70826. Cancer Med. 2025. PMID: 40186416 Free PMC article.
Cited by
-
Immune Checkpoint Inhibitors in "Special" NSCLC Populations: A Viable Approach?Int J Mol Sci. 2023 Aug 9;24(16):12622. doi: 10.3390/ijms241612622. Int J Mol Sci. 2023. PMID: 37628803 Free PMC article. Review.
-
Current status and progress of PD-L1 detection: guiding immunotherapy for non-small cell lung cancer.Clin Exp Med. 2024 Jul 18;24(1):162. doi: 10.1007/s10238-024-01404-1. Clin Exp Med. 2024. PMID: 39026109 Free PMC article. Review.
-
Granzyme B and melittin in cancer immunotherapy: molecular mechanisms and therapeutic perspectives in head and neck cancers.Front Immunol. 2025 Jul 22;16:1628014. doi: 10.3389/fimmu.2025.1628014. eCollection 2025. Front Immunol. 2025. PMID: 40766321 Free PMC article. Review.
-
NSCLC: from tumorigenesis, immune checkpoint misuse to current and future targeted therapy.Front Immunol. 2024 Feb 7;15:1342086. doi: 10.3389/fimmu.2024.1342086. eCollection 2024. Front Immunol. 2024. PMID: 38384472 Free PMC article. Review.
-
From rough to precise: PD-L1 evaluation for predicting the efficacy of PD-1/PD-L1 blockades.Front Immunol. 2022 Aug 3;13:920021. doi: 10.3389/fimmu.2022.920021. eCollection 2022. Front Immunol. 2022. PMID: 35990664 Free PMC article. Review.
References
-
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. . Five-Year Survival and Correlates Among Patients With Advanced Melanoma, Renal Cell Carcinoma, or Non-Small Cell Lung Cancer Treated With Nivolumab. JAMA Oncol (2019) 5(10):1411–20. 10.1001/jamaoncol.2019.2187 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

