KLF4, a Key Regulator of a Transitive Triplet, Acts on the TGF-β Signaling Pathway and Contributes to High-Altitude Adaptation of Tibetan Pigs
- PMID: 33936161
- PMCID: PMC8082500
- DOI: 10.3389/fgene.2021.628192
KLF4, a Key Regulator of a Transitive Triplet, Acts on the TGF-β Signaling Pathway and Contributes to High-Altitude Adaptation of Tibetan Pigs
Abstract
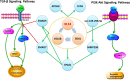
Tibetan pigs are native mammalian species on the Tibetan Plateau that have evolved distinct physiological traits that allow them to tolerate high-altitude hypoxic environments. However, the genetic mechanism underlying this adaptation remains elusive. Here, based on multitissue transcriptional data from high-altitude Tibetan pigs and low-altitude Rongchang pigs, we performed a weighted correlation network analysis (WGCNA) and identified key modules related to these tissues. Complex network analysis and bioinformatics analysis were integrated to identify key genes and three-node network motifs. We found that among the six tissues (muscle, liver, heart, spleen, kidneys, and lungs), lung tissue may be the key organs for Tibetan pigs to adapt to hypoxic environment. In the lung tissue of Tibetan pigs, we identified KLF4, BCL6B, EGR1, EPAS1, SMAD6, SMAD7, KDR, ATOH8, and CCN1 genes as potential regulators of hypoxia adaption. We found that KLF4 and EGR1 genes might simultaneously regulate the BCL6B gene, forming a KLF4-EGR1-BCL6B complex. This complex, dominated by KLF4, may enhance the hypoxia tolerance of Tibetan pigs by mediating the TGF-β signaling pathway. The complex may also affect the PI3K-Akt signaling pathway, which plays an important role in angiogenesis caused by hypoxia. Therefore, we postulate that the KLF4-EGR1-BCL6B complex may be beneficial for Tibetan pigs to survive better in the hypoxia environments. Although further molecular experiments and independent large-scale studies are needed to verify our findings, these findings may provide new details of the regulatory architecture of hypoxia-adaptive genes and are valuable for understanding the genetic mechanism of hypoxic adaptation in mammals.
Keywords: Tibetan pig; gene network; hypoxia adaptation; multitissue; transcriptome.
Copyright © 2021 Wang, Guo, Liu, Zhang, Cui, Ding, Wang, Wang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Screening of functional genes for hypoxia adaptation in Tibetan pigs by combined genome resequencing and transcriptome analysis.Front Vet Sci. 2024 Oct 21;11:1486258. doi: 10.3389/fvets.2024.1486258. eCollection 2024. Front Vet Sci. 2024. PMID: 39497743 Free PMC article.
-
Gene Co-Expression Network Analysis Unraveling Transcriptional Regulation of High-Altitude Adaptation of Tibetan Pig.PLoS One. 2016 Dec 9;11(12):e0168161. doi: 10.1371/journal.pone.0168161. eCollection 2016. PLoS One. 2016. PMID: 27936142 Free PMC article.
-
The Expression Regulatory Network in the Lung Tissue of Tibetan Pigs Provides Insight Into Hypoxia-Sensitive Pathways in High-Altitude Hypoxia.Front Genet. 2021 Oct 7;12:691592. doi: 10.3389/fgene.2021.691592. eCollection 2021. Front Genet. 2021. PMID: 34691141 Free PMC article.
-
Tibetan and Andean patterns of adaptation to high-altitude hypoxia.Hum Biol. 2000 Feb;72(1):201-28. Hum Biol. 2000. PMID: 10721618 Review.
-
Energy power in mountains: difference in metabolism pattern results in different adaption traits in Tibetans.Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2012 Nov;28(6):488-93. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2012. PMID: 23581177 Review.
Cited by
-
Hypoxia-induced cancer cell reprogramming: a review on how cancer stem cells arise.Front Oncol. 2023 Aug 8;13:1227884. doi: 10.3389/fonc.2023.1227884. eCollection 2023. Front Oncol. 2023. PMID: 37614497 Free PMC article. Review.
-
Hypoxia-inducible factor-1α attenuates renal podocyte injury in male rats in a simulated high-altitude environment by upregulating Krüppel-like factor 4 expression.Exp Physiol. 2024 Jul;109(7):1188-1198. doi: 10.1113/EP091443. Epub 2024 May 22. Exp Physiol. 2024. PMID: 38774964 Free PMC article.
-
Enhancing oxygen utilization and mitigating oxidative stress in Tibetan chickens for adaptation to high-altitude hypoxia.Poult Sci. 2025 Apr;104(4):104893. doi: 10.1016/j.psj.2025.104893. Epub 2025 Feb 7. Poult Sci. 2025. PMID: 40014967 Free PMC article.
-
Integrative Genomic, Transcriptomic and Epigenomic Analysis Reveals cis-regulatory Contributions to High-altitude Adaptation in Tibetan Pigs.Mol Biol Evol. 2025 Jul 1;42(7):msaf169. doi: 10.1093/molbev/msaf169. Mol Biol Evol. 2025. PMID: 40644385 Free PMC article.
-
Divergence of Liver Lipidomes in Tibetan and Yorkshire Pigs Living at Different Altitudes.Molecules. 2023 Mar 27;28(7):2991. doi: 10.3390/molecules28072991. Molecules. 2023. PMID: 37049754 Free PMC article.
References
-
- Ambalavanan N., Nicola T., Hagood J., Bulger A., Serra R., Murphy-Ullrich J., et al. (2008). Transforming growth factor-beta signaling mediates hypoxia-induced pulmonary arterial remodeling and inhibition of alveolar development in newborn mouse lung. Am. J. Physiol. Lung. Cell. Mol. Physiol. 295 L86–L95. 10.1152/ajplung.00534.2007 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

