Complete response to larotrectinib treatment in a patient with papillary thyroid cancer harboring an ETV6-NTRK3 gene fusion
- PMID: 33936613
- PMCID: PMC8077291
- DOI: 10.1002/ccr3.3900
Complete response to larotrectinib treatment in a patient with papillary thyroid cancer harboring an ETV6-NTRK3 gene fusion
Abstract
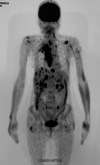
Larotrectinib, a highly selective TRK inhibitor, was administered to a patient with rapidly progressing radioactive iodine-refractory papillary NTRK3 fusion-positive thyroid cancer. The patient achieved a durable (sustained for 11 months) complete response after 2 months of treatment and complete intracranial responses in metastatic brain lesions after 7 months of treatment. Larotrectinib may provide a therapeutic route for patients with RAI-R-differentiated thyroid cancer who might otherwise have few treatment options.
Keywords: iodine uptake and iodine metabolism; thyroid cancer‐clinical; thyroid cancer‐genetics.
© 2021 The Authors. Clinical Case Reports published by John Wiley & Sons Ltd.
Conflict of interest statement
Fabián Pitoia discloses speaker bureau/expert testimony fees/honoraria from Bayer, Exelixis, Sanofi, Grupo Biotoscana (Representative of EISAI in Argentina), and Raffo Laboratory.
Figures





References
-
- James BC, Mitchell JM, Jeon HD, Vasilottos N, Grogan RH, Aschebrook‐Kilfoy B. An update in international trends in incidence rates of thyroid cancer, 1973–2007. Cancer Causes Control. 2018;29:465‐473. - PubMed
-
- Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140:317‐322. - PubMed
-
- Kim J, Gosnell JE, Roman SA. Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol. 2020;16:17‐29. - PubMed
-
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1‐133. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

