The crystal structures, Hirshfeld surface analyses and energy frameworks of 8-{1-[3-(cyclo-pent-1-en-1-yl)benz-yl]piperidin-4-yl}-2-meth-oxy-quinoline and 8-{4-[3-(cyclo-pent-1-en-1-yl)benz-yl]piperazin-1-yl}-2-meth-oxy-quinoline
- PMID: 33936760
- PMCID: PMC8025868
- DOI: 10.1107/S2056989021002474
The crystal structures, Hirshfeld surface analyses and energy frameworks of 8-{1-[3-(cyclo-pent-1-en-1-yl)benz-yl]piperidin-4-yl}-2-meth-oxy-quinoline and 8-{4-[3-(cyclo-pent-1-en-1-yl)benz-yl]piperazin-1-yl}-2-meth-oxy-quinoline
Abstract
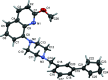
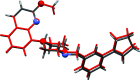
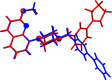
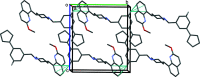
The title compounds, 8-{1-[3-(cyclo-pent-1-en-1-yl)benz-yl]piperidin-4-yl}-2-meth-oxy-quinoline, C27H30N2O (I), and 8-{4-[3-(cyclo-pent-1-en-1-yl)benz-yl]piperazin-1-yl}-2-meth-oxy-quinoline, C26H29N3O (II), differ only in the nature of the central six-membered ring: piperidine in I and piperazine in II. They are isoelectronic (CH cf. N) and isotypic; they both crystallize in the triclinic space group P with very similar unit-cell parameters. Both mol-ecules have a curved shape and very similar conformations. In the biaryl group, the phenyl ring is inclined to the cyclo-pentene mean plane (r.m.s. deviations = 0.089 Å for I and 0.082 Å for II) by 15.83 (9) and 13.82 (6)° in I and II, respectively, and by 67.68 (6) and 69.47 (10)°, respectively, to the mean plane of the quinoline moiety (r.m.s. deviations = 0.034 Å for I and 0.038 Å for II). The piperazine ring in I and the piperidine ring in II have chair conformations. In the crystals of both compounds, mol-ecules are linked by C-H⋯π inter-actions, forming chains in I and ribbons in II, both propagating along the b-axis direction. The principal contributions to the overall Hirshfeld surfaces involve H⋯H contacts at 67.5 and 65.9% for I and II, respectively. The major contribution to the inter-molecular inter-actions in the crystals is from dispersion forces (E dis), reflecting the absence of classical hydrogen bonds.
Keywords: Hirshfeld surface analysis; crystal structure; cyclopentene; dopamine D2; energy frameworks; hydrogen bonding; isoelectronic; isotypic; methoxyquinoline; piperazine; piperidine; serotonin 5-HT1a.
© Ullah and Stoeckli-Evans 2021.
Figures




References
-
- Cosi, C., Carilla-Durand, E., Assié, M.-B., Ormiere, A. M., Maraval, M., Leduc, N. & Newman-Tancredi, A. (2006). Eur. J. Pharmacol. 535, 135–144. - PubMed
-
- Feenstra, R. W., de Moes, J., Hofma, J. J., Kling, H., Kuipers, W., Long, S. K., Tulp, M. T. M., van der Heyden, J. A. M. & Kruse, C. G. (2001). Bioorg. Med. Chem. Lett. 11, 2345–2349. - PubMed
-
- Feenstra, R. W., van den Hoogenband, A., Stroomer, C. N. J., van Stuivenberg, H. H., Tulp, M. T. M., Long, S. K., van der Heyden, J. A. M. & Kruse, C. G. (2006). Chem. Pharm. Bull. 54, 1326–1330. - PubMed
-
- Ghani, U., Ullah, N., Ali, S. A. & Al-Muallem, H. A. (2014). Asian J. Chem. 26, 8258–8362.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
