LCT-3d Induces Oxidative Stress-Mediated Apoptosis by Upregulating Death Receptor 5 in Gastric Cancer Cells
- PMID: 33937072
- PMCID: PMC8085419
- DOI: 10.3389/fonc.2021.658608
LCT-3d Induces Oxidative Stress-Mediated Apoptosis by Upregulating Death Receptor 5 in Gastric Cancer Cells
Erratum in
-
Corrigendum: LCT-3d induces oxidative stress-mediated apoptosis by upregulating death receptor 5 in gastric cancer cells.Front Oncol. 2025 May 29;15:1590809. doi: 10.3389/fonc.2025.1590809. eCollection 2025. Front Oncol. 2025. PMID: 40510138 Free PMC article.
Abstract
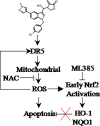
Gastric cancer is a global health problem. In this study, we investigate the role of a novel Indole derivative, named LCT-3d, in inhibiting the growth of gastric cancer cells by MTT assay. The Western blotting results showed that LCT-3d modulated the mitochondrial-related proteins and Cleaved-Caspases 3/9, to induce cell apoptosis. The up-regulation of Death receptor 5 (DR5) in MGC803 cells was observed with LCT-3d treatment. Knockdown of DR5 on MGC803 cells partially reversed the LCT-3d-induced mitochondrial apoptosis. The level of Reactive Oxygen Species (ROS) in MGC803 cells was increased with LCT-3d treatment and could be blocked with the pretreatment of the ROS inhibitor N-Acetylcysteine (NAC). The results demonstrate that the elevating ROS can up-regulate the expression of DR5, resulting in apoptosis via mitochondrial pathway. Although the nuclear factor erythroid-2 related factor 2 (Nrf2) pathway served an important role in protecting gastric cancer cells against the injury of ROS, it can't reverse LCT-3d-induced cell apoptosis. Taken together, our study showed that LCT-3d induced apoptosis via DR5-mediated mitochondrial apoptotic pathway in gastric cancer cells. LCT-3d could be a novel lead compound for development of anti-cancer activity in gastric cancer.
Keywords: DR5; LCT-3d; Nrf2; apoptosis; gastric cancer; reactive oxygen species.
Copyright © 2021 Wang, Wu, Yu, Hu, Li, Meng, Lv, Kim, Choi, Wang, Xu and Jin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer JH declared a shared affiliation with one of the authors, G-YK, to the handling editor at time of review.
Figures






References
-
- Shen X, Wang J, Yan X, Ren X, Wang F, Chen X, et al. Predictive value of GSTP1 Ile105Val polymorphism in clinical outcomes of chemotherapy in gastric and colorectal cancers: a systematic review and meta-analysis. Cancer Chemother Pharmacol (2016) 77:1285–302. 10.1007/s00280-016-3047-1 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

