The Redox-Active Tyrosine Is Essential for Proton Pumping in Cytochrome c Oxidase
- PMID: 33937193
- PMCID: PMC8079940
- DOI: 10.3389/fchem.2021.640155
The Redox-Active Tyrosine Is Essential for Proton Pumping in Cytochrome c Oxidase
Abstract
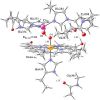
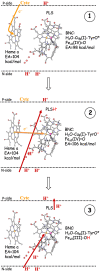
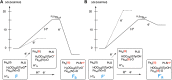
Cellular respiration involves electron transport via a number of enzyme complexes to the terminal Cytochrome c oxidase (CcO), in which molecular oxygen is reduced to water. The free energy released in the reduction process is used to establish a transmembrane electrochemical gradient, via two processes, both corresponding to charge transport across the membrane in which the enzymes are embedded. First, the reduction chemistry occurring in the active site of CcO is electrogenic, which means that the electrons and protons are delivered from opposite sides of the membrane. Second, the exergonic chemistry is coupled to translocation of protons across the entire membrane, referred to as proton pumping. In the largest subfamily of the CcO enzymes, the A-family, one proton is pumped for every electron needed for the chemistry, making the energy conservation particularly efficient. In the present study, hybrid density functional calculations are performed on a model of the A-family CcOs. The calculations show that the redox-active tyrosine, conserved in all types of CcOs, plays an essential role for the energy conservation. Based on the calculations a reaction mechanism is suggested involving a tyrosyl radical (possibly mixed with tyrosinate character) in all reduction steps. The result is that the free energy released in each reduction step is large enough to allow proton pumping in all reduction steps without prohibitively high barriers when the gradient is present. Furthermore, the unprotonated tyrosine provides a mechanism for coupling the uptake of two protons per electron in every reduction step, i.e. for a secure proton pumping.
Keywords: cytochrome c oxidase; density functional theory; energy conservation; midpoint potentials; proton pumping; redox-active tyrosine.
Copyright © 2021 Blomberg.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Becke A. D. (1993). Density‐functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652. 10.1063/1.464913 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

