Modular Approaches to Synthesize Activity- and Affinity-Based Chemical Probes
- PMID: 33937194
- PMCID: PMC8082414
- DOI: 10.3389/fchem.2021.644811
Modular Approaches to Synthesize Activity- and Affinity-Based Chemical Probes
Abstract
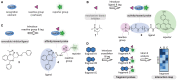
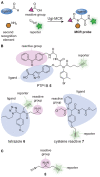
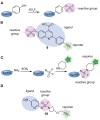
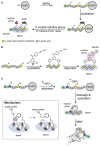
Combinatorial and modular methods to synthesize small molecule modulators of protein activity have proven to be powerful tools in the development of new drug-like molecules. Over the past decade, these methodologies have been adapted toward utilization in the development of activity- and affinity-based chemical probes, as well as in chemoproteomic profiling. In this review, we will discuss how methods like multicomponent reactions, DNA-encoded libraries, phage displays, and others provide new ways to rapidly screen novel chemical probes against proteins of interest.
Keywords: DNA-templated; SuFEx; activity-based probe; affinity-based probe; chemical probe design; iminoboronate chemistry; protein profiling.
Copyright © 2021 van der Zouwen and Witte.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Iminoboronates as Dual-Purpose Linkers in Chemical Probe Development.Chemistry. 2021 Feb 15;27(10):3292-3296. doi: 10.1002/chem.202005115. Epub 2021 Jan 14. Chemistry. 2021. PMID: 33259638 Free PMC article.
-
DNA-Encoded Dynamic Combinatorial Chemical Libraries.Angew Chem Int Ed Engl. 2015 Jun 26;54(27):7924-8. doi: 10.1002/anie.201501775. Epub 2015 May 26. Angew Chem Int Ed Engl. 2015. PMID: 26014116
-
20 years of DNA-encoded chemical libraries.Chem Commun (Camb). 2011 Dec 28;47(48):12747-53. doi: 10.1039/c1cc15634a. Epub 2011 Nov 14. Chem Commun (Camb). 2011. PMID: 22083211 Review.
-
DNA-encoded chemical libraries: advancing beyond conventional small-molecule libraries.Acc Chem Res. 2014 Apr 15;47(4):1247-55. doi: 10.1021/ar400284t. Epub 2014 Mar 28. Acc Chem Res. 2014. PMID: 24673190
-
Activity-based protein profiling: A graphical review.Curr Res Pharmacol Drug Discov. 2023 Aug 24;5:100164. doi: 10.1016/j.crphar.2023.100164. eCollection 2023. Curr Res Pharmacol Drug Discov. 2023. PMID: 37692766 Free PMC article. Review.
Cited by
-
The Evolution of Fluorescein into A Potential Theranostic Tool.Chemistry. 2025 Jun 17;31(34):e202501513. doi: 10.1002/chem.202501513. Epub 2025 May 13. Chemistry. 2025. PMID: 40317604 Free PMC article.
-
Chemical Proteomics Reveals Off-Targets of the Anandamide Reuptake Inhibitor WOBE437.ACS Chem Biol. 2022 May 20;17(5):1174-1183. doi: 10.1021/acschembio.2c00122. Epub 2022 Apr 28. ACS Chem Biol. 2022. PMID: 35482948 Free PMC article.
-
bioTCIs: Middle-to-Macro Biomolecular Targeted Covalent Inhibitors Possessing Both Semi-Permanent Drug Action and Stringent Target Specificity as Potential Antibody Replacements.Int J Mol Sci. 2023 Feb 9;24(4):3525. doi: 10.3390/ijms24043525. Int J Mol Sci. 2023. PMID: 36834935 Free PMC article. Review.
-
Target identification of small molecules: an overview of the current applications in drug discovery.BMC Biotechnol. 2023 Oct 10;23(1):44. doi: 10.1186/s12896-023-00815-4. BMC Biotechnol. 2023. PMID: 37817108 Free PMC article. Review.
-
The linkage-type and the exchange molecule affect the protein-labeling efficiency of iminoboronate probes.Org Biomol Chem. 2023 Nov 29;21(46):9173-9181. doi: 10.1039/d3ob01269g. Org Biomol Chem. 2023. PMID: 37947354 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

