Synthesis Candidates Herbicide Through Optimization Quinclorac Containing 3-Methyl-1H-pyrazol-5-yl
- PMID: 33937195
- PMCID: PMC8080966
- DOI: 10.3389/fchem.2021.647472
Synthesis Candidates Herbicide Through Optimization Quinclorac Containing 3-Methyl-1H-pyrazol-5-yl
Abstract
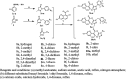
To enhance quinclorac potency, twenty-five derivatives were synthesized containing 3-methyl-1H-pyrazol-5-yl by intermediate derivatization methods (IDMs). These compounds were confirmed by melting point (mp), 1HNMR, 13CNMR, and HRMS. The compound 1,3-dimethyl-1H-pyrazol-5-yl 3,7-dichloroquinoline-8-carboxylate (10a) was determined by X-ray diffraction. The activity of these compounds substituent on the phenyl was: electron-drawing group > neutral group > donor-drawing group, the results was like that of substituted benzyl group on pyrazole. The herbicidal activity assays showed that compounds 1-(2-fluorophenyl)-3-methyl-1H-pyrazol-5-yl 3,7-dichloroquinoline-8-carboxylate (8l, EC50 = 10.53 g/ha) and 10a (EC50 = 10.37 g/ha) had an excellent inhibition effect on barnyard grass in greenhouse experiment. Greenhouse safety experiment of rice exhibited almost no difference in plant height and fresh weight treated 10a at stage 1∼2-leaf of rice after 14 days but 8l had a detrimental effect. Two season field assays showed 10a herbicidal activity on barnyard grass at 150 g/ha as equal as 300 g/ha quinclorac in fields in 2019 and 2020. The study demonstrated that 10a could be further researched as a potential herbicide to control barnyard grass in fields.
Keywords: barnyard grass; field assay; herbicidal activity; intermediate derivatization methods (IDMs); quinclorac.
Copyright © 2021 Luo, Bai, Zhou, Wu, Zhang, Wu, Li and Bai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Belmar J., Alderete J., Zuñiga C., Jiménez C., Jiménez V., Núñez H., et al. (2001). Synthesis of 1-n-Alkyl-3-Methyl- and 1-n-Alkyl-3- Phenyl-5-Pyrazolones and formyl derivatives. Bol. Soc. Chil. Quím. 46, 459–470. 10.4067/S0366-16442001000400010 - DOI
-
- Burris E., Leonard B. R., Martin S. H., White C. A., Graves J. B., Shaw R., et al. (1994). Fipronil: evaluation of soil and foliar treatments for control of thrips, aphids, plant bugs and boll weevils. Proceedings 2, 838–844.
-
- Cole D., Pallett K., Rodgers M. (2000). Discovering new modes of action for herbicides and the impact of genomics. Pesti. Outlook 11, 223–229. 10.1039/b009272j - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

