New Quinolinone O-GlcNAc Transferase Inhibitors Based on Fragment Growth
- PMID: 33937202
- PMCID: PMC8079942
- DOI: 10.3389/fchem.2021.666122
New Quinolinone O-GlcNAc Transferase Inhibitors Based on Fragment Growth
Abstract
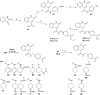
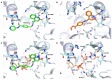
O-GlcNAcylation is an important post-translational and metabolic process in cells that must be carefully regulated. O-GlcNAc transferase (OGT) is ubiquitously present in cells and is the only enzyme that catalyzes the transfer of O-GlcNAc to proteins. OGT is a promising target in various pathologies such as cancer, immune system diseases, or nervous impairment. In our previous work we identified the 2-oxo-1,2-dihydroquinoline-4-carboxamide derivatives as promising compounds by a fragment-based drug design approach. Herein, we report the extension of this first series with several new fragments. As the most potent fragment, we identified 3b with an IC50 value of 116.0 μM. If compared with the most potent inhibitor of the first series, F20 (IC50 = 117.6 μM), we can conclude that the new fragments did not improve OGT inhibition remarkably. Therefore, F20 was used as the basis for the design of a series of compounds with the elongation toward the O-GlcNAc binding pocket as the free carboxylate allows easy conjugation. Compound 6b with an IC50 value of 144.5 μM showed the most potent OGT inhibition among the elongated compounds, but it loses inhibition potency when compared to the UDP mimetic F20. We therefore assume that the binding of the compounds in the O-GlcNAc binding pocket is likely not crucial for OGT inhibition. Furthermore, evaluation of the compounds with two different assays revealed that some inhibitors most likely interfere with the commercially available UDP-Glo™ glycosyltransferase assay, leading to false positive results. This observation calls for caution, when evaluating UDP mimetic as OGT inhibitors with the UDP-Glo™ glycosyltransferase assay, as misinterpretations can occur.
Keywords: O-GlcNAc; O-GlcNAc transferase; fragments growth; molecular docking; protein glycosylation.
Copyright © 2021 Weiss, Loi, Sterle, Balsollier, Tomašič, Pieters, Gobec and Anderluh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

