Adverse Health-Related Quality of Life Outcome Despite Adequate Clinical Response to Treatment in Systemic Lupus Erythematosus
- PMID: 33937290
- PMCID: PMC8085308
- DOI: 10.3389/fmed.2021.651249
Adverse Health-Related Quality of Life Outcome Despite Adequate Clinical Response to Treatment in Systemic Lupus Erythematosus
Abstract
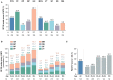
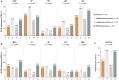
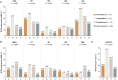
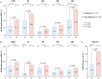
Objective: To determine the prevalence of adverse health-related quality of life (HRQoL) outcomes in patients with SLE who achieved an adequate clinical response after a 52-week long standard therapy plus belimumab or placebo, and identify contributing factors. Methods: We included patients who met the primary endpoint of the BLISS-52 (NCT00424476) and BLISS-76 (NCT00410384) trials, i.e., SLE Responder Index 4 (total population: N = 760/1,684; placebo: N = 217/562; belimumab 1 mg/kg: N = 258/559; belimumab 10 mg/kg: N = 285/563). Adverse HRQoL outcomes were defined as SF-36 scale scores ≤ the 5th percentile derived from age- and sex-matched population-based norms, and FACIT-Fatigue scores <30. We investigated factors associated with adverse HRQoL outcomes using logistic regression analysis. Results: We found clinically important diminutions of HRQoL in SLE patients compared with matched norms and high frequencies of adverse HRQoL outcomes, the highest in SF-36 general health (29.1%), followed by FACIT-Fatigue (25.8%) and SF-36 physical functioning (25.4%). Overall, frequencies were higher with increasing age. Black/African American and White/Caucasian patients reported higher frequencies than Asians and Indigenous Americans, while Hispanics experienced adverse HRQoL outcome less frequently than non-Hispanics. Established organ damage was associated with adverse physical but not mental HRQoL outcomes; particularly, damage in the cardiovascular (OR: 2.12; 95% CI: 1.07-4.21; P = 0.032) and musculoskeletal (OR: 1.41; 95% CI: 1.01-1.96; P = 0.041) domains was associated with adverse SF-36 physical component summary. Disease activity showed no impact on HRQoL outcomes. In multivariable logistic regression analysis, addition of belimumab to standard therapy was associated with lower frequencies of adverse SF-36 physical functioning (OR: 0.59; 95% CI: 0.39-0.91; P = 0.016) and FACIT-F (OR: 0.53; 95% CI: 0.34-0.81; P = 0.004). Conclusions: Despite adequate clinical response to standard therapy plus belimumab or placebo, a substantial proportion of SLE patients still reported adverse HRQoL outcomes. While no impact was documented for disease activity, established organ damage contributed to adverse outcome within physical HRQoL aspects and add-on belimumab was shown to be protective against adverse physical functioning and severe fatigue.
Keywords: biologic drugs; fatigue; health-related quality of life; patient perscpective; patient-reported outcome; systemic lupus erythematosus.
Copyright © 2021 Gomez, Qiu, Cederlund, Borg, Lindblom, Emamikia, Enman, Lampa and Parodis.
Conflict of interest statement
IP has received research funding and/or honoraria from Amgen, Elli Lilly and Company, Gilead Sciences, GlaxoSmithKline and Novartis. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Jolly M. How does quality of life of patients with systemic lupus erythematosus compare with that of other common chronic illnesses? J Rheumatol. (2005) 32:1706–8. - PubMed
-
- Strand V, Levy RA, Cervera R, Petri MA, Birch H, Freimuth WW, et al. Improvements in health-related quality of life with belimumab, a B-lymphocyte stimulator-specific inhibitor, in patients with autoantibody-positive systemic lupus erythematosus from the randomised controlled BLISS trials. Ann Rheum Dis. (2014) 73:838–44. 10.1136/annrheumdis-2012-202865 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

