TRIM21, a New Component of the TRAIL-Induced Endogenous Necrosome Complex
- PMID: 33937329
- PMCID: PMC8082149
- DOI: 10.3389/fmolb.2021.645134
TRIM21, a New Component of the TRAIL-Induced Endogenous Necrosome Complex
Abstract
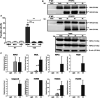
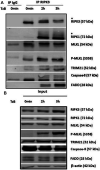
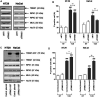
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a well-known apoptosis inducer and a potential anticancer agent. When caspases and inhibitors of apoptosis proteins (IAPs) are inhibited, TRAIL induces necroptosis. Molecular mechanisms of necroptosis rely on kinase activation, and on the formation of a necrosome complex, bringing together the receptor-interacting protein kinases 1 and 3 (RIPK1, RIPK3), and the mixed lineage kinase domain-like protein (MLKL). In this study, mass spectrometry approach allowed to identify the tripartite motif containing 21 (TRIM21), an E3 ubiquitin-protein ligase as a new partner of the endogenous TRAIL-induced necrosome. Alteration of TRIM21 expression level, obtained by transient transfection of HT29 or HaCat cells with TRIM21-targeted siRNAs or cDNA plasmids coding for TRIM21 demonstrated that TRIM21 is a positive regulator of TRAIL-induced necroptosis. Furthermore, the invalidation of TRIM21 expression in HT29 cells by CRISPR-Cas9 technology also decreased cell sensitivity to TRAIL-induced necroptosis, a shortcoming associated with a reduction in MLKL phosphorylation, the necroptosis executioner. Thus, TRIM21 emerged as a new partner of the TRAIL-induced necrosome that positively regulates the necroptosis process.
Keywords: TRAIL; TRIM21; necroptosis; necrosome; proteomics.
Copyright © 2021 Simoes Eugénio, Faurez, Kara-Ali, Lagarrigue, Uhart, Bonnet, Gallais, Com, Pineau, Samson, Le Seyec and Dimanche-Boitrel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

