Protein Aggregation as a Bacterial Strategy to Survive Antibiotic Treatment
- PMID: 33937340
- PMCID: PMC8085434
- DOI: 10.3389/fmolb.2021.669664
Protein Aggregation as a Bacterial Strategy to Survive Antibiotic Treatment
Abstract
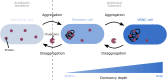
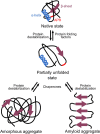
While protein aggregation is predominantly associated with loss of function and toxicity, it is also known to increase survival of bacteria under stressful conditions. Indeed, protein aggregation not only helps bacteria to cope with proteotoxic stresses like heat shocks or oxidative stress, but a growing number of studies suggest that it also improves survival during antibiotic treatment by inducing dormancy. A well-known example of dormant cells are persisters, which are transiently refractory to the action of antibiotics. These persister cells can switch back to the susceptible state and resume growth in the absence of antibiotics, and are therefore considered an important cause of recurrence of infections. Mounting evidence now suggests that this antibiotic-tolerant persister state is tightly linked to-or perhaps even driven by-protein aggregation. Moreover, another dormant bacterial phenotype, the viable but non-culturable (VBNC) state, was also shown to be associated with aggregation. These results indicate that persisters and VBNC cells may constitute different stages of the same dormancy program induced by progressive protein aggregation. In this mini review, we discuss the relation between aggregation and bacterial dormancy, focusing on both persisters and VBNC cells. Understanding the link between protein aggregation and dormancy will not only provide insight into the fundamentals of bacterial survival, but could prove highly valuable in our future battle to fight them.
Keywords: VBNC; amorphous aggregate; amyloid; antibiotic tolerance; dormancy; persistence; stress response.
Copyright © 2021 Bollen, Dewachter and Michiels.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

