Resilience to anhedonia-passive coping induced by early life experience is linked to a long-lasting reduction of Ih current in VTA dopaminergic neurons
- PMID: 33937445
- PMCID: PMC8079665
- DOI: 10.1016/j.ynstr.2021.100324
Resilience to anhedonia-passive coping induced by early life experience is linked to a long-lasting reduction of Ih current in VTA dopaminergic neurons
Abstract
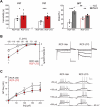
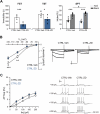
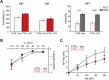
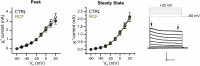
Exposure to aversive events during sensitive developmental periods can affect the preferential coping strategy adopted by individuals later in life, leading to either stress-related psychiatric disorders, including depression, or to well-adaptation to future adversity and sources of stress, a behavior phenotype termed "resilience". We have previously shown that interfering with the development of mother-pups bond with the Repeated Cross Fostering (RCF) stress protocol can induce resilience to depression-like phenotype in adult C57BL/6J female mice. Here, we used patch-clamp recording in midbrain slice combined with both in vivo and ex vivo pharmacology to test our hypothesis of a link between electrophysiological modifications of dopaminergic neurons in the intermediate Ventral Tegmental Area (VTA) of RCF animals and behavioral resilience. We found reduced hyperpolarization-activated (Ih) cation current amplitude and evoked firing in VTA dopaminergic neurons from both young and adult RCF female mice. In vivo, VTA-specific pharmacological manipulation of the Ih current reverted the pro-resilient phenotype in adult early-stressed mice or mimicked behavioral resilience in adult control animals. This is the first evidence showing how pro-resilience behavior induced by early events is linked to a long-lasting reduction of Ih current and excitability in VTA dopaminergic neurons.
Keywords: Early stress; Ih current; In vivo pharmacology; Resilience; VTA.
© 2021 The Author(s).
Conflict of interest statement
None.
Figures



References
-
- American Psychiatric Association . fifth ed. 2013. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC.
-
- Andolina D., Di Segni M., Accoto A., Iacono L.L., Borreca A., Ielpo D., Berretta N., Perlas E., Puglisi-Allegra S., Ventura R. MicroRNA-34 contributes to the stress-related behavior and affects 5-HT prefrontal/GABA amygdalar system through regulation of corticotropin-releasing factor receptor 1. Mol. Neurobiol. 2018;55:7401–7412. - PubMed
-
- Authement M.E., Kodangattil J.N., Gouty S., Rusnak M., Symes A.J., Cox B.M., Nugent F.S. Histone deacetylase inhibition rescues maternal deprivation-induced GABAergic metaplasticity through restoration of AKAP signaling. Neuron. 2015;86:1240–1252. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

